
6
December

By Katie Gerhards
There’s more than one way to kill bacteria—and in a world of increasing antibiotic resistance, researchers need to uncover as many ways as possible. The National Institutes of Health is helping Assistant Professor Jason Peters hit the ground running in pursuit of that goal with his new research program in the University of Wisconsin–Madison School of Pharmacy’s Pharmaceutical Sciences Division.
The NIH National Institute of Allergy and Infectious Diseases awarded Peters a K22 Career Transition grant to acquire equipment and extra hands for his lab, which is focused on finding new genetic targets to kill bacteria, thus curbing the impacts of antibiotic resistance.
The grant is designed to help promising young researchers like Peters make the transition from postdoctoral scholars to faculty members, who need to establish their labs and require flexible funding to adapt and grow.
Peters, who joined the School’s Drug Action Core in August, will be developing CRISPR-based tools to uncover genetic functions crucial to the life and reproduction of pathogenic bacteria. As a postdoc, he developed new CRISPRi strategies to perturb essential genes in benign bacteria, but now he will be expanding that technology to target pathogens that are difficult to study using genetics.
Antibiotics work by disrupting bacteria’s essential functions, preventing them from continuing to live and propagate an infection. But the problem is that most antibiotics target the same essential functions, and bacteria are increasingly finding ways to protect those functions.
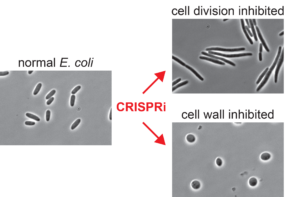
“A lot of the antibiotics that we use kill bacteria in very similar ways. And what is really important for treatment in the face is resistance is to find new mechanisms to kill the bacteria,” explains Peters. “I’m particularly pushing into antibiotic-resistant pathogens because we really need to understand how they work so that we can understand how to fight them.”
Using his CRISPR tools, he is aiming to find previously unknown essential functions and then collaborate with colleagues within the School, such as Professor Tim Bugni in the Pharmaceutical Science Division’s Drug Discovery Core and Associate Professor Warren Rose in the Pharmacy Practice Division to translate those new targets into new medication therapies. Similarly, Peters can work backward, and examine an existing or new antibiotic to see how it works and which function it’s targeting.
With the K22 grant, Peters says he can expand the scope of projects he’d already considered. Instead of doing CRISPR screens for three pathogens, for example, he can add several more and accelerate his results with additional help and equipment.
“Having the ability to expand into a larger set of bacteria could be huge,” says Peters. “It could mean the difference between learning the important function that differentiates the pathogen from the model bacteria or missing that opportunity altogether.”
So far, the grant has enabled Peters to add an assistant scientist, Amy Banta, to his group and acquire custom designed pieces of DNA used to generate CRISPR strains.
“The flexibility this grant provides is amazing,” he says. “There won’t be any hesitation moving forward.”
Learn more about Peters’ research, his CRISPRi tools, and ongoing projects in the Peters Lab.




