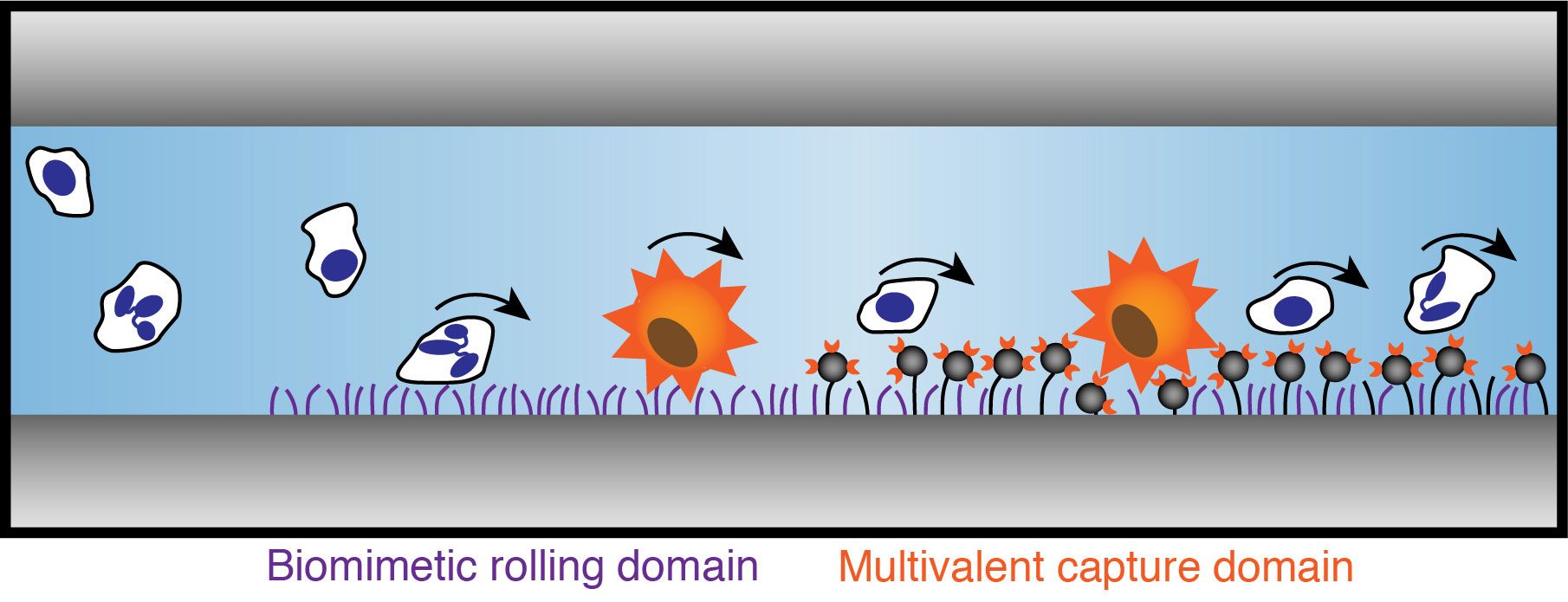
Metastatic cancer colonies are initiated with an adhesion mechanism hijacked from the inflammation response. The first step in this process involves transient, reversible, adhesive interactions between selectin molecules expressed on the endothelial venules and glycoprotein receptors on the leukocyte/invasive cancer cell, resulting in cell rolling. By exploiting this natural process, circulating tumor cells (CTCs) may be selectively captured on devices containing selectins and other cancer cell specific ligands. To translate this technique to realistic devices, capturing surfaces need to be non-fouling, stable, and controllable with minimum batch-by-batch variations, which are major drawbacks of current immobilization methods of the ligands, i.e. physisorption. Covalent immobilization of cell-specific ligands using non-toxic chemical reactions can enhance these properties as compared to physisorbed surfaces both in model and in vitro studies.
The results suggest that cancer cell specific capturing devices based on cell rolling can be achieved if the ligands are covalently immobilized in a controlled way and present high specificity against their targets. An optimized, polymer-based nanovector is employed for this application in order to enhance the specific capturing capacity of the devices via multivalent effects.