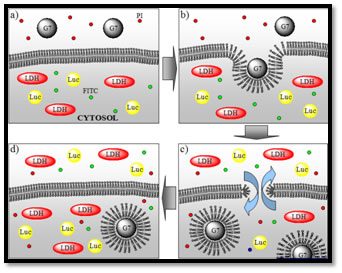
In order to develop effective delivery systems, it is essential to develop a more fundamental understanding of nanomaterial-cell interactions. We have found that positively-charged polyamidoamine (PAMAM) dendrimers and other polycationic polymers induce the formation of transient pores in cellular membranes. This phenomenon plays a key role in cellular uptake of these materials, competing with endocytic mechanisms of uptake.
Efficient target tissue permeation is crucial for achieving desired drug responses. Dense biological tissues and their complex localized matrices present significant barriers towards efficient nanoparticle delivery. Using dendrimers, we have studied the effect of nanoparticle size and surface charge on transport into multicellular tumor spheroids. By precisely tuning the size and charge of dendrimers, we are able to control their penetration and accumulation within the spheroids, with smaller generation dendrimers penetrating to the spheroid core, and larger dendrimers accumulating on the periphery of the spheroids. Moreover, simple surface charge modifications dictate the accumulation of dendrimers within the spheroids. We have also found that dendrimer penetration through skin layers is similarly dependent on both the dendrimer size and surface charge. Our findings illuminate how nanoparticle size, surface charge, and functionalization can be tuned for highly controlled biodistributions.
