Tumor-derived blood-circulating exosomes have potential as a biomarker to greatly improve cancer treatment. However, effective isolation of exosomes remains a tremendous technical challenge. This study presents a novel nanostructured polymer surface for highly effective capture of exosomes through strong avidity.
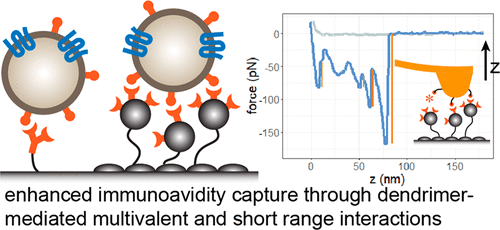
Various surface configurations, consisting of multivalent dendrimers, PEG, and tumor-targeting antibodies, were tested using exosomes isolated from tumor cell lines. We found that a dual layer dendrimer configuration exhibited the highest efficiency in capturing cultured exosomes spiked into human serum. Importantly, the optimized surface captured a > 4-fold greater amount of tumor exosomes from head and neck cancer patient plasma samples than that from healthy donors. Nanomechanical analysis using atomic force microscopy also revealed that the enhancement was attributed to multivalent binding (avidity) and augmented short-range adhesion mediated by dendrimers. Our results support that the dendrimer surface detects tumor exosomes at high sensitivity and specificity, demonstrating its potential as a new cancer liquid biopsy platform.
This article is cited by 16 publications