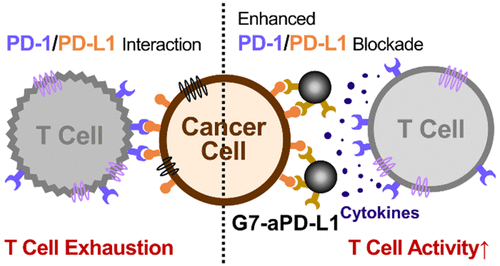
Upregulation of programmed death ligand 1 (PD-L1) allows cancer cells to evade antitumor immunity. Despite tremendous efforts in developing PD-1/PD-L1 immune checkpoint inhibitors (ICIs), clinical trials using such ICIs have shown inconsistent benefits. Here, we hypothesized that the ICI efficacy would be dictated by the binding strength of the inhibitor to the target proteins. To assess this, hyperbranched, multivalent poly(amidoamine) dendrimers were employed to prepare dendrimer–ICI conjugates (G7-aPD-L1). Binding kinetics measurements using SPR, BLI, and AFM revealed that G7-aPD-L1 exhibits significantly enhanced binding strength to PD-L1 proteins, compared to free aPD-L1. The binding avidity of G7-aPD-L1 was translated into in vitro efficiency and in vivo selectivity, as the conjugates improved the PD-L1 blockade effect and enhanced accumulation in tumor sites. Our results demonstrate that the dendrimer-mediated multivalent interaction substantially increases the binding avidity of the ICIs and thereby improves the antagonist effect, providing a novel platform for cancer immunotherapy.
This article is cited by 19 publications