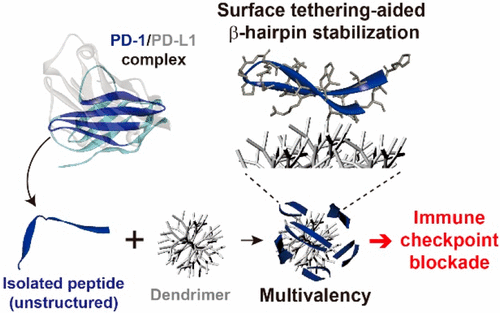
β-Hairpin peptides present great potential as antagonists against β-sheet-rich protein surfaces, of which wide and flat geometries are typically “undruggable” with small molecules. Herein, we introduce a peptide–dendrimer conjugate (PDC) approach that stabilizes the β-hairpin structure of the peptide via intermolecular forces and the excluded volume effect as well as exploits the multivalent binding effect. Because of the synergistic advantages, the PDCs based on a β-hairpin peptide isolated from an engineered programmed death-1 (PD-1) protein showed significantly higher affinity (avidity) to their binding counterpart, programmed death-ligand 1 (PD-L1), as compared to free peptides (by up to 5 orders of magnitude). The enhanced binding kinetics with high selectivity was translated into an improved immune checkpoint inhibitory effect in vitro, at a level comparable to (if not better than) that of a full-size monoclonal antibody. The results demonstrate the potential of the PDC system as a novel class of inhibitors targeting β-strand-rich protein surfaces, such as PD-1 and PD-L1, displaying its potential as a new cancer immunotherapy platform.
This article is cited by 11 publications