
By Will Cushman
This article originally appeared on UW–Madison’s news page.
A newly developed nanomaterial that mimics the behavior of proteins could be an effective tool for treating Alzheimer’s and other neurodegenerative diseases. The nanomaterial alters the interaction between two key proteins in brain cells — with a potentially powerful therapeutic effect.
The innovative findings, recently published in the journal Advanced Materials, were made possible thanks to a collaboration between University of Wisconsin–Madison scientists and nanomaterial engineers at Northwestern University.
The work centers around altering the interaction between two proteins that are believed to be involved in setting the stage for diseases like Alzheimer’s, Parkinson’s and amyotrophic lateral sclerosis, or ALS.
The first protein is called Nrf2, a specific type of protein called a transcription factor that turns genes on and off within cells.
One of Nrf2’s important functions is its antioxidant effect. While different neurodegenerative diseases result from separate disease processes, a commonality among them is the toxic effect of oxidative stress on neurons and other nerve cells. Nrf2 combats this toxic stress in brain cells, helping to stave off disease.
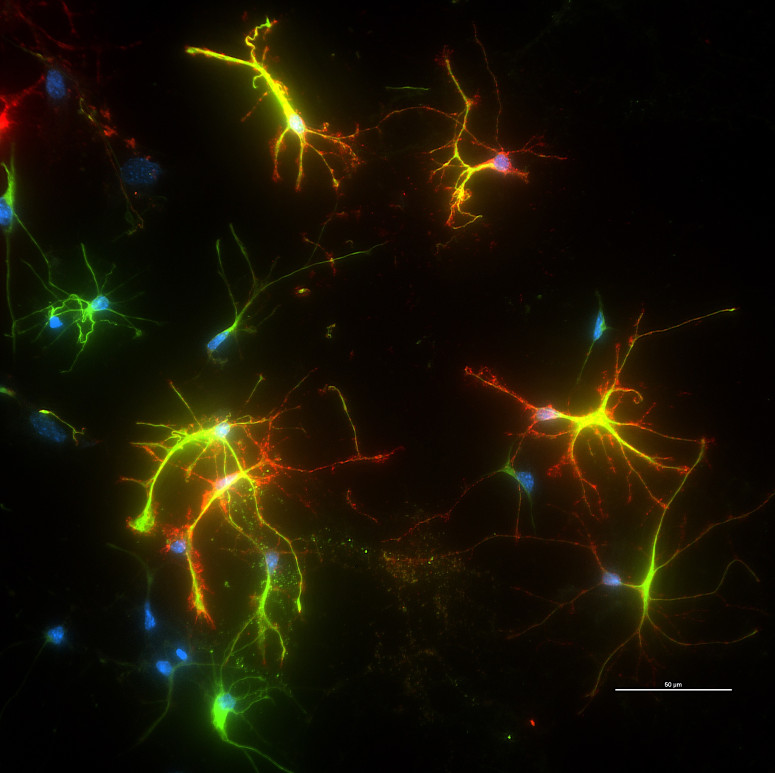
Jeffrey Johnson, a professor in the UW–Madison School of Pharmacy, has been studying Nrf2 as a promising target for treating neurodegenerative diseases for decades alongside his wife, Delinda Johnson, a senior scientist at the pharmacy school. In 2022, the Johnsons and another group of collaborators found that increasing Nrf2 activity in a specific cell type in the brain, the astrocyte, helped protect neurons in mouse models of Alzheimer’s disease, leading to significantly less memory loss.
While this previous research suggested that increasing Nrf2’s activity could form the basis of an Alzheimer’s treatment, scientists have found it challenging to effectively target the protein within the brain.
“It’s hard to get drugs into the brain, but it’s also been very hard to find drugs that activate Nrf2 without a lot of off-target effects,” says Jeffrey Johnson.
Enter the new nanomaterial. Known as a protein-like polymer, or PLP, the synthetic material is designed to bind to proteins as if it were itself a protein. This nano-scale imitator is a product of a team led by Nathan Gianneschi, a professor of chemistry at Northwestern and faculty member at the university’s International Institute for Nanotechnology.
Gianneschi has designed multiple PLPs to target various proteins. This particular PLP is structured to alter the interaction between Nrf2 and another protein called Keap1. The proteins’ interaction, or pathway, is a well-known target for treating many conditions because Keap1 essentially controls when Nrf2 responds to — and combats — oxidative stress. Bound together under unstressed conditions, Keap1 releases Nrf2 to do its antioxidant work when needed.
Gianneschi and the Johnsons were connected via Robert Pacifici, chief science officer at the CHDI Foundation, which funds research aimed at treating Huntington’s disease, another neurodegenerative condition. The foundation has funded both the Johnsons’ and Gianneschi’s work in the past.

“Just in passing, Nathan and his colleagues at Grove Biopharma, a preclinical biotech startup focused on therapeutic targeting of protein-protein interactions, said to Robert that they were thinking about moving to target Nrf2,” says Johnson. “And Robert said, ‘If you’re going to do that, you should call Jeff Johnson.’”
Soon, the Johnsons and Gianneschi were discussing the possibility of the UW–Madison lab providing mouse model brain cells needed to test Gianneschi’s protein-like nanomaterial.
Jeffrey Johnson says he was initially somewhat skeptical about the PLP approach, given his unfamiliarity with it and the general difficulty of precisely targeting proteins in brain cells.
“But then one of Nathan’s students came up here with it and put it on our cells, and I’ll be damned if it didn’t work really well,” he says. “We really dove into it then.”
The resulting research showed that Gianneschi’s PLP was very effective at binding to Keap1, which freed up Nrf2 to accumulate in cells’ nuclei, amping up its antioxidant function. Importantly, it did so without causing the unwanted off-target effects that have hampered other strategies aimed at better activating Nrf2.
While that work was performed in cells in culture, the Johnsons and Gianneschi are now taking it a step further in mouse models of neurodegenerative diseases. It’s a line of research that they hadn’t expected to be involved in but are now excited to be pursuing.
“We don’t have the expertise in biomaterials,” says Delinda Johnson. “So getting that from Northwestern and then moving forward on the biological side here at UW shows that these types of collaborations are really important.”

