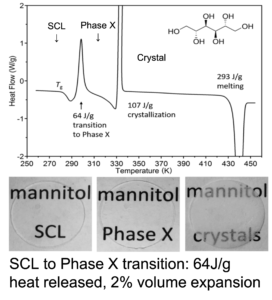
Polyamorphism refers to the existence of two amorphous phases of the same composition separated by a firstorder transition. This phenomenon is analogous to crystal polymorphism exemplified by diamond and graphite, but is less well understood and even controversial. Polyamorphism occurs in the extensive hydrogen-bonded polyalcohol D-mannitol, C6H8(OH)6. The low-temperature-stable second amorphous phase has lower density, lower energy and stronger hydrogen bonds. X-ray scattering and solid-state NMR help us determine the different amorphous structures. This provides molecular-level insight into the ability to modify glass structures and create new materials with better stability and performance.