Abstract
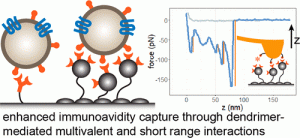
Tumor-derived blood-circulating exosomes have potential as a biomarker to greatly improve cancer treatment. However, effective isolation of exosomes remains a tremendous technical challenge. This study presents a novel nanostructured polymer surface for highly effective capture of exosomes through strong avidity.
Various surface configurations, consisting of multivalent dendrimers, PEG, and tumor-targeting antibodies, were tested using exosomes isolated from tumor cell lines. We found that a dual layer dendrimer configuration exhibited the highest efficiency in capturing cultured exosomes spiked into human serum. Importantly, the optimized surface captured a > 4-fold greater amount of tumor exosomes from head and neck cancer patient plasma samples than that from healthy donors. Nanomechanical analysis using atomic force microscopy also revealed that the enhancement was attributed to multivalent binding (avidity) and augmented short-range adhesion mediated by dendrimers. Our results support that the dendrimer surface detects tumor exosomes at high sensitivity and specificity, demonstrating its potential as a new cancer liquid biopsy platform.
Cited by
This article is cited by 16 publications
-
Poellmann, M. J., Bu, J., Liu, S., Wang, A. Z., Seyedin, S. N., Chandrasekharan, C., Hong, H., Kim, Y., Caster, J. M., & Hong, S. (2023). Nanotechnology and machine learning enable circulating tumor cells as a reliable biomarker for radiotherapy responses of gastrointestinal cancer patients.
Biosensors & bioelectronics,
226, 115117.
https://doi.org/10.1016/j.bios.2023.115117
-
Yin, Y., Han, X., Li, C., Sun, T., Li, K., Liu, X., & Liu, M. (2022). The status of industrialization and development of exosomes as a drug delivery system: A review.
Frontiers in pharmacology,
13, 961127.
https://doi.org/10.3389/fphar.2022.961127
-
Poellmann, M. J., Rawding, P., Kim, D., Bu, J., Kim, Y., & Hong, S. (2022). Branched, dendritic, and hyperbranched polymers in liquid biopsy device design.
Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology,
14(3), e1770.
https://doi.org/10.1002/wnan.1770
-
Lee, T., Rawding, P. A., Bu, J., Hyun, S., Rou, W., Jeon, H., Kim, S., Lee, B., Kubiatowicz, L. J., Kim, D., Hong, S., & Eun, H. (2022). Machine-Learning-Based Clinical Biomarker Using Cell-Free DNA for Hepatocellular Carcinoma (HCC).
Cancers,
14(9), 2061.
https://doi.org/10.3390/cancers14092061
-
Rawding, P. A., Bu, J., Wang, J., Kim, D. W., Drelich, A. J., Kim, Y., & Hong, S. (2022). Dendrimers for cancer immunotherapy: Avidity-based drug delivery vehicles for effective anti-tumor immune response.
Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology,
14(2), e1752.
https://doi.org/10.1002/wnan.1752
-
Jeong, W. J., Bu, J., Jafari, R., Rehak, P., Kubiatowicz, L. J., Drelich, A. J., Owen, R. H., Nair, A., Rawding, P. A., Poellmann, M. J., Hopkins, C. M., Král, P., & Hong, S. (2022). Hierarchically Multivalent Peptide-Nanoparticle Architectures: A Systematic Approach to Engineer Surface Adhesion.
Advanced science (Weinheim, Baden-Wurttemberg, Germany),
9(4), e2103098.
https://doi.org/10.1002/advs.202103098
-
Nair, A., Bu, J., Rawding, P. A., Do, S. C., Li, H., & Hong, S. (2021). Cytochalasin B Treatment and Osmotic Pressure Enhance the Production of Extracellular Vesicles (EVs) with Improved Drug Loading Capacity.
Nanomaterials (Basel, Switzerland),
12(1), 3.
https://doi.org/10.3390/nano12010003
-
Saad, M. G., Beyenal, H., & Dong, W. J. (2021). Exosomes as Powerful Engines in Cancer: Isolation, Characterization and Detection Techniques.
Biosensors,
11(12), 518.
https://doi.org/10.3390/bios11120518
-
Min, L., Wang, B., Bao, H., Li, X., Zhao, L., Meng, J., & Wang, S. (2021). Advanced Nanotechnologies for Extracellular Vesicle-Based Liquid Biopsy.
Advanced science (Weinheim, Baden-Wurttemberg, Germany),
8(20), e2102789.
https://doi.org/10.1002/advs.202102789
-
Nair, A., Bu, J., Bugno, J., Rawding, P. A., Kubiatowicz, L. J., Jeong, W. J., & Hong, S. (2021). Size-Dependent Drug Loading, Gene Complexation, Cell Uptake, and Transfection of a Novel Dendron-Lipid Nanoparticle for Drug/Gene Co-delivery.
Biomacromolecules,
22(9), 3746–3755.
https://doi.org/10.1021/acs.biomac.1c00541
-
Wang, J., Drelich, A. J., Hopkins, C. M., Mecozzi, S., Li, L., Kwon, G., & Hong, S. (2021). Gold nanoparticles in virus detection: Recent advances and potential considerations for SARS-CoV-2 testing development.
Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology, e1754. Advance online publication.
https://doi.org/10.1002/wnan.1754
-
Cheng, S., Li, Y., Yan, H., Wen, Y., Zhou, X., Friedman, L., & Zeng, Y. (2021). Advances in microfluidic extracellular vesicle analysis for cancer diagnostics.
Lab on a chip,
21(17), 3219–3243.
https://doi.org/10.1039/d1lc00443c
-
Bu, J., Lee, T. H., Poellmann, M. J., Rawding, P. A., Jeong, W. J., Hong, R. S., Hyun, S. H., Eun, H. S., & Hong, S. (2021). Tri-modal liquid biopsy: Combinational analysis of circulating tumor cells, exosomes, and cell-free DNA using machine learning algorithm.
Clinical and translational medicine,
11(8), e499.
https://doi.org/10.1002/ctm2.499
-
Herrmann, M., Diederichs, S., Melnik, S., Riegger, J., Trivanović, D., Li, S., Jenei-Lanzl, Z., Brenner, R. E., Huber-Lang, M., Zaucke, F., Schildberg, F. A., & Grässel, S. (2021). Extracellular Vesicles in Musculoskeletal Pathologies and Regeneration.
Frontiers in bioengineering and biotechnology,
8, 624096.
https://doi.org/10.3389/fbioe.2020.624096
-
Bu, J., Lee, T. H., Jeong, W. J., Poellmann, M. J., Mudd, K., Eun, H. S., Liu, E. W., Hong, S., & Hyun, S. H. (2020). Enhanced detection of cell-free DNA (cfDNA) enables its use as a reliable biomarker for diagnosis and prognosis of gastric cancer.
PloS one,
15(12), e0242145.
https://doi.org/10.1371/journal.pone.0242145
-
Bu, J., Nair, A., Iida, M., Jeong, W. J., Poellmann, M. J., Mudd, K., Kubiatowicz, L. J., Liu, E. W., Wheeler, D. L., & Hong, S. (2020). An Avidity-Based PD-L1 Antagonist Using Nanoparticle-Antibody Conjugates for Enhanced Immunotherapy.
Nano letters,
20(7), 4901–4909.
https://doi.org/10.1021/acs.nanolett.0c00953
Read the full article at:
https://pubs.acs.org/action/showCitFormats?doi=10.1021%2Facs.nanolett.0c00950&href=/doi/10.1021%2Facs.nanolett.0c00950
