Abstract
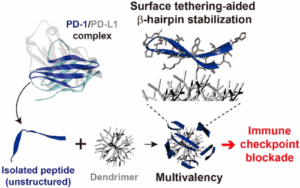
β-Hairpin peptides present great potential as antagonists against β-sheet-rich protein surfaces, of which wide and flat geometries are typically “undruggable” with small molecules. Herein, we introduce a peptide–dendrimer conjugate (PDC) approach that stabilizes the β-hairpin structure of the peptide via intermolecular forces and the excluded volume effect as well as exploits the multivalent binding effect. Because of the synergistic advantages, the PDCs based on a β-hairpin peptide isolated from an engineered programmed death-1 (PD-1) protein showed significantly higher affinity (avidity) to their binding counterpart, programmed death-ligand 1 (PD-L1), as compared to free peptides (by up to 5 orders of magnitude). The enhanced binding kinetics with high selectivity was translated into an improved immune checkpoint inhibitory effect in vitro, at a level comparable to (if not better than) that of a full-size monoclonal antibody. The results demonstrate the potential of the PDC system as a novel class of inhibitors targeting β-strand-rich protein surfaces, such as PD-1 and PD-L1, displaying its potential as a new cancer immunotherapy platform.
Cited by
This article is cited by 11 publications-
Gu, Z., Xu, S., Guo, Z., & Liu, Z. (2022). Rational development of molecularly imprinted nanoparticles for blocking PD-1/PD-L1 axis. Chemical science, 13(36), 10897–10903. https://doi.org/10.1039/d2sc03412c
-
Poellmann, M. J., Rawding, P., Kim, D., Bu, J., Kim, Y., & Hong, S. (2022). Branched, dendritic, and hyperbranched polymers in liquid biopsy device design. Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology, 14(3), e1770. https://doi.org/10.1002/wnan.1770
-
Rawding, P. A., Bu, J., Wang, J., Kim, D. W., Drelich, A. J., Kim, Y., & Hong, S. (2022). Dendrimers for cancer immunotherapy: Avidity-based drug delivery vehicles for effective anti-tumor immune response. Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology, 14(2), e1752. https://doi.org/10.1002/wnan.1752
-
Jeong, W. J., Bu, J., Jafari, R., Rehak, P., Kubiatowicz, L. J., Drelich, A. J., Owen, R. H., Nair, A., Rawding, P. A., Poellmann, M. J., Hopkins, C. M., Král, P., & Hong, S. (2022). Hierarchically Multivalent Peptide-Nanoparticle Architectures: A Systematic Approach to Engineer Surface Adhesion. Advanced science (Weinheim, Baden-Wurttemberg, Germany), 9(4), e2103098. https://doi.org/10.1002/advs.202103098
-
Ershov, P. V., Mezentsev, Y. V., & Ivanov, A. S. (2022). Interfacial Peptides as Affinity Modulating Agents of Protein-Protein Interactions. Biomolecules, 12(1), 106. https://doi.org/10.3390/biom12010106
-
Nair, A., Bu, J., Bugno, J., Rawding, P. A., Kubiatowicz, L. J., Jeong, W. J., & Hong, S. (2021). Size-Dependent Drug Loading, Gene Complexation, Cell Uptake, and Transfection of a Novel Dendron-Lipid Nanoparticle for Drug/Gene Co-delivery. Biomacromolecules, 22(9), 3746–3755. https://doi.org/10.1021/acs.biomac.1c00541
-
Liu, C., Seeram, N. P., & Ma, H. (2021). Small molecule inhibitors against PD-1/PD-L1 immune checkpoints and current methodologies for their development: a review. Cancer cell international, 21(1), 239. https://doi.org/10.1186/s12935-021-01946-4
-
Li, L., Ma, B., & Wang, W. (2020). Peptide-Based Nanomaterials for Tumor Immunotherapy. Molecules (Basel, Switzerland), 26(1), 132. https://doi.org/10.3390/molecules26010132
-
Skibba, M., Drelich, A., Poellmann, M., Hong, S., & Brasier, A. R. (2020). Nanoapproaches to Modifying Epigenetics of Epithelial Mesenchymal Transition for Treatment of Pulmonary Fibrosis. Frontiers in pharmacology, 11, 607689. https://doi.org/10.3389/fphar.2020.607689
-
Bu, J., Lee, T. H., Jeong, W. J., Poellmann, M. J., Mudd, K., Eun, H. S., Liu, E. W., Hong, S., & Hyun, S. H. (2020). Enhanced detection of cell-free DNA (cfDNA) enables its use as a reliable biomarker for diagnosis and prognosis of gastric cancer. PloS one, 15(12), e0242145. https://doi.org/10.1371/journal.pone.0242145
-
Bu, J., Nair, A., Iida, M., Jeong, W. J., Poellmann, M. J., Mudd, K., Kubiatowicz, L. J., Liu, E. W., Wheeler, D. L., & Hong, S. (2020). An Avidity-Based PD-L1 Antagonist Using Nanoparticle-Antibody Conjugates for Enhanced Immunotherapy. Nano letters, 20(7), 4901–4909. https://doi.org/10.1021/acs.nanolett.0c00953