Abstract
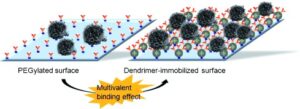
A naturally occurring multivalent binding effect is manipulated by engineering cell capture surfaces using dendrimers. The enhanced binding through the multivalent effect significantly improves detection of tumor cells. This improvement can be potentially translated into clinically significant detection of circulating tumor cells from the blood of cancer patients.
Read the full article at:
https://onlinelibrary.wiley.com/doi/10.1002/anie.201105508

Cited by
This article is cited by 55 publications-
Poellmann, M. J., Bu, J., Liu, S., Wang, A. Z., Seyedin, S. N., Chandrasekharan, C., Hong, H., Kim, Y., Caster, J. M., & Hong, S. (2023). Nanotechnology and machine learning enable circulating tumor cells as a reliable biomarker for radiotherapy responses of gastrointestinal cancer patients. Biosensors & bioelectronics, 226, 115117. https://doi.org/10.1016/j.bios.2023.115117
-
Poellmann, M. J., Rawding, P., Kim, D., Bu, J., Kim, Y., & Hong, S. (2022). Branched, dendritic, and hyperbranched polymers in liquid biopsy device design. Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology, 14(3), e1770. https://doi.org/10.1002/wnan.1770
-
Rawding, P. A., Bu, J., Wang, J., Kim, D. W., Drelich, A. J., Kim, Y., & Hong, S. (2022). Dendrimers for cancer immunotherapy: Avidity-based drug delivery vehicles for effective anti-tumor immune response. Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology, 14(2), e1752. https://doi.org/10.1002/wnan.1752
-
Jeong, W. J., Bu, J., Jafari, R., Rehak, P., Kubiatowicz, L. J., Drelich, A. J., Owen, R. H., Nair, A., Rawding, P. A., Poellmann, M. J., Hopkins, C. M., Král, P., & Hong, S. (2022). Hierarchically Multivalent Peptide-Nanoparticle Architectures: A Systematic Approach to Engineer Surface Adhesion. Advanced science (Weinheim, Baden-Wurttemberg, Germany), 9(4), e2103098. https://doi.org/10.1002/advs.202103098
-
Nair, A., Bu, J., Bugno, J., Rawding, P. A., Kubiatowicz, L. J., Jeong, W. J., & Hong, S. (2021). Size-Dependent Drug Loading, Gene Complexation, Cell Uptake, and Transfection of a Novel Dendron-Lipid Nanoparticle for Drug/Gene Co-delivery. Biomacromolecules, 22(9), 3746–3755. https://doi.org/10.1021/acs.biomac.1c00541
-
Wu, J., Long, Y., Li, M., & He, Q. (2021). Emerging nanomedicine-based therapeutics for hematogenous metastatic cascade inhibition: Interfering with the crosstalk between “seed and soil”. Acta pharmaceutica Sinica. B, 11(8), 2286–2305. https://doi.org/10.1016/j.apsb.2020.11.024
-
Qin, W., Chen, L., Wang, Z., Li, Q., Fan, C., Wu, M., & Zhang, Y. (2020). Bioinspired DNA Nanointerface with Anisotropic Aptamers for Accurate Capture of Circulating Tumor Cells. Advanced science (Weinheim, Baden-Wurttemberg, Germany), 7(19), 2000647. https://doi.org/10.1002/advs.202000647
-
Bu, J., Nair, A., Iida, M., Jeong, W. J., Poellmann, M. J., Mudd, K., Kubiatowicz, L. J., Liu, E. W., Wheeler, D. L., & Hong, S. (2020). An Avidity-Based PD-L1 Antagonist Using Nanoparticle-Antibody Conjugates for Enhanced Immunotherapy. Nano letters, 20(7), 4901–4909. https://doi.org/10.1021/acs.nanolett.0c00953
-
Dong, J., Chen, J. F., Smalley, M., Zhao, M., Ke, Z., Zhu, Y., & Tseng, H. R. (2020). Nanostructured Substrates for Detection and Characterization of Circulating Rare Cells: From Materials Research to Clinical Applications. Advanced materials (Deerfield Beach, Fla.), 32(1), e1903663. https://doi.org/10.1002/adma.201903663
-
Iliescu, F. S., Poenar, D. P., Yu, F., Ni, M., Chan, K. H., Cima, I., Taylor, H. K., Cima, I., & Iliescu, C. (2019). Recent advances in microfluidic methods in cancer liquid biopsy. Biomicrofluidics, 13(4), 041503. https://doi.org/10.1063/1.5087690
-
Myung, J. H., Cha, A., Tam, K. A., Poellmann, M., Borgeat, A., Sharifi, R., Molokie, R. E., Votta-Velis, G., & Hong, S. (2019). Dendrimer-Based Platform for Effective Capture of Tumor Cells after TGFβ1-Induced Epithelial-Mesenchymal Transition. Analytical chemistry, 91(13), 8374–8382. https://doi.org/10.1021/acs.analchem.9b01181
-
Gribko, A., Künzel, J., Wünsch, D., Lu, Q., Nagel, S. M., Knauer, S. K., Stauber, R. H., & Ding, G. B. (2019). Is small smarter? Nanomaterial-based detection and elimination of circulating tumor cells: current knowledge and perspectives. International journal of nanomedicine, 14, 4187–4209. https://doi.org/10.2147/IJN.S198319
-
Xu, J., Wang, X., Yan, C., & Chen, W. (2019). A Polyamidoamine Dendrimer-Based Electrochemical Immunosensor for Label-Free Determination of Epithelial Cell Adhesion Molecule- Expressing Cancer Cells. Sensors (Basel, Switzerland), 19(8), 1879. https://doi.org/10.3390/s19081879
-
Myung, J. H., Eblan, M. J., Caster, J. M., Park, S. J., Poellmann, M. J., Wang, K., Tam, K. A., Miller, S. M., Shen, C., Chen, R. C., Zhang, T., Tepper, J. E., Chera, B. S., Wang, A. Z., & Hong, S. (2018). Multivalent Binding and Biomimetic Cell Rolling Improves the Sensitivity and Specificity of Circulating Tumor Cell Capture. Clinical cancer research : an official journal of the American Association for Cancer Research, 24(11), 2539–2547. https://doi.org/10.1158/1078-0432.CCR-17-3078
-
Myung, J. H., Park, S. J., Wang, A. Z., & Hong, S. (2018). Integration of biomimicry and nanotechnology for significantly improved detection of circulating tumor cells (CTCs). Advanced drug delivery reviews, 125, 36–47. https://doi.org/10.1016/j.addr.2017.12.005
-
Jan, Y. J., Chen, J. F., Zhu, Y., Lu, Y. T., Chen, S. H., Chung, H., Smalley, M., Huang, Y. W., Dong, J., Chen, L. C., Yu, H. H., Tomlinson, J. S., Hou, S., Agopian, V. G., Posadas, E. M., & Tseng, H. R. (2018). NanoVelcro rare-cell assays for detection and characterization of circulating tumor cells. Advanced drug delivery reviews, 125, 78–93. https://doi.org/10.1016/j.addr.2018.03.006
-
Shen, M. Y., Chen, J. F., Luo, C. H., Lee, S., Li, C. H., Yang, Y. L., Tsai, Y. H., Ho, B. C., Bao, L. R., Lee, T. J., Jan, Y. J., Zhu, Y. Z., Cheng, S., Feng, F. Y., Chen, P., Hou, S., Agopian, V., Hsiao, Y. S., Tseng, H. R., Posadas, E. M., … Yu, H. H. (2018). Glycan Stimulation Enables Purification of Prostate Cancer Circulating Tumor Cells on PEDOT NanoVelcro Chips for RNA Biomarker Detection. Advanced healthcare materials, 7(3), 10.1002/adhm.201700701. https://doi.org/10.1002/adhm.201700701
-
Zhang, W., Wang, J., Li, P., Wu, C., Zhang, H., Zhang, W., Wang, H., & Tang, B. (2017). Transferrin-navigation Nano Artificial Antibody Fluorescence Recognition of Circulating Tumor Cells. Scientific reports, 7(1), 10142. https://doi.org/10.1038/s41598-017-10486-9
-
Song, Y., Tian, T., Shi, Y., Liu, W., Zou, Y., Khajvand, T., Wang, S., Zhu, Z., & Yang, C. (2017). Enrichment and single-cell analysis of circulating tumor cells. Chemical science, 8(3), 1736–1751. https://doi.org/10.1039/c6sc04671a
-
Zhang, Z., & King, M. R. (2017). Nanomaterials for the Capture and Therapeutic Targeting of Circulating Tumor Cells. Cellular and molecular bioengineering, 10(4), 275–294. https://doi.org/10.1007/s12195-017-0497-4
-
Jiang, X., Bugno, J., Hu, C., Yang, Y., Herold, T., Qi, J., Chen, P., Gurbuxani, S., Arnovitz, S., Strong, J., Ferchen, K., Ulrich, B., Weng, H., Wang, Y., Huang, H., Li, S., Neilly, M. B., Larson, R. A., Le Beau, M. M., Bohlander, S. K., … Chen, J. (2016). Eradication of Acute Myeloid Leukemia with FLT3 Ligand-Targeted miR-150 Nanoparticles. Cancer research, 76(15), 4470–4480. https://doi.org/10.1158/0008-5472.CAN-15-2949
-
Pearson, R. M., Sen, S., Hsu, H. J., Pasko, M., Gaske, M., Král, P., & Hong, S. (2016). Tuning the Selectivity of Dendron Micelles Through Variations of the Poly(ethylene glycol) Corona. ACS nano, 10(7), 6905–6914. https://doi.org/10.1021/acsnano.6b02708
-
Chen, J. F., Zhu, Y., Lu, Y. T., Hodara, E., Hou, S., Agopian, V. G., Tomlinson, J. S., Posadas, E. M., & Tseng, H. R. (2016). Clinical Applications of NanoVelcro Rare-Cell Assays for Detection and Characterization of Circulating Tumor Cells. Theranostics, 6(9), 1425–1439. https://doi.org/10.7150/thno.15359
-
Myung, J. H., Tam, K. A., Park, S. J., Cha, A., & Hong, S. (2016). Recent advances in nanotechnology-based detection and separation of circulating tumor cells. Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology, 8(2), 223–239. https://doi.org/10.1002/wnan.1360
-
Myung, J. H., & Hong, S. (2015). Microfluidic devices to enrich and isolate circulating tumor cells. Lab on a chip, 15(24), 4500–4511. https://doi.org/10.1039/c5lc00947b
-
Myung, J. H., Roengvoraphoj, M., Tam, K. A., Ma, T., Memoli, V. A., Dmitrovsky, E., Freemantle, S. J., & Hong, S. (2015). Effective capture of circulating tumor cells from a transgenic mouse lung cancer model using dendrimer surfaces immobilized with anti-EGFR. Analytical chemistry, 87(19), 10096–10102. https://doi.org/10.1021/acs.analchem.5b02766
-
Mitchell, M. J., Castellanos, C. A., & King, M. R. (2015). Immobilized surfactant-nanotube complexes support selectin-mediated capture of viable circulating tumor cells in the absence of capture antibodies. Journal of biomedical materials research. Part A, 103(10), 3407–3418. https://doi.org/10.1002/jbm.a.35445
-
Li, S., Chen, N., Gaddes, E. R., Zhang, X., Dong, C., & Wang, Y. (2015). A Drosera-bioinspired hydrogel for catching and killing cancer cells. Scientific reports, 5, 14297. https://doi.org/10.1038/srep14297
-
Sharma, A., & Kakkar, A. (2015). Designing Dendrimer and Miktoarm Polymer Based Multi-Tasking Nanocarriers for Efficient Medical Therapy. Molecules (Basel, Switzerland), 20(9), 16987–17015. https://doi.org/10.3390/molecules200916987
-
Bugno, J., Hsu, H. J., & Hong, S. (2015). Recent advances in targeted drug delivery approaches using dendritic polymers. Biomaterials science, 3(7), 1025–1034. https://doi.org/10.1039/c4bm00351a
-
Tang, L., Yin, Q., Xu, Y., Zhou, Q., Cai, K., Yen, J., Dobrucki, L. W., & Cheng, J. (2015). Bioorthogonal Oxime Ligation Mediated In Vivo Cancer Targeting. Chemical science, 6(4), 2182–2186. https://doi.org/10.1039/C5SC00063G
-
Xie, J., Wang, J., Chen, H., Shen, W., Sinko, P. J., Dong, H., Zhao, R., Lu, Y., Zhu, Y., & Jia, L. (2015). Multivalent conjugation of antibody to dendrimers for the enhanced capture and regulation on colon cancer cells. Scientific reports, 5, 9445. https://doi.org/10.1038/srep09445
-
Xie, J., Dong, H., Chen, H., Zhao, R., Sinko, P. J., Shen, W., Wang, J., Lu, Y., Yang, X., Xie, F., & Jia, L. (2015). Exploring cancer metastasis prevention strategy: interrupting adhesion of cancer cells to vascular endothelia of potential metastatic tissues by antibody-coated nanomaterial. Journal of nanobiotechnology, 13, 9. https://doi.org/10.1186/s12951-015-0072-x
-
Bugno, J., Hsu, H. J., & Hong, S. (2015). Tweaking dendrimers and dendritic nanoparticles for controlled nano-bio interactions: potential nanocarriers for improved cancer targeting. Journal of drug targeting, 23(7-8), 642–650. https://doi.org/10.3109/1061186X.2015.1052077
-
Wang, S., Wan, Y., & Liu, Y. (2014). Effects of nanopillar array diameter and spacing on cancer cell capture and cell behaviors. Nanoscale, 6(21), 12482–12489. https://doi.org/10.1039/c4nr02854f
-
Xie, J., Zhao, R., Gu, S., Dong, H., Wang, J., Lu, Y., Sinko, P. J., Yu, T., Xie, F., Wang, L., Shao, J., & Jia, L. (2014). The architecture and biological function of dual antibody-coated dendrimers: enhanced control of circulating tumor cells and their hetero-adhesion to endothelial cells for metastasis prevention. Theranostics, 4(12), 1250–1263. https://doi.org/10.7150/thno.8775
-
Tang, Y., Shi, J., Li, S., Wang, L., Cayre, Y. E., & Chen, Y. (2014). Microfluidic device with integrated microfilter of conical-shaped holes for high efficiency and high purity capture of circulating tumor cells. Scientific reports, 4, 6052. https://doi.org/10.1038/srep06052
-
Zhang, J., Sheng, W., & Fan, Z. H. (2014). An ensemble of aptamers and antibodies for multivalent capture of cancer cells. Chemical communications (Cambridge, England), 50(51), 6722–6725. https://doi.org/10.1039/c4cc02002b
-
Myung, J. H., Gajjar, K. A., Chen, J., Molokie, R. E., & Hong, S. (2014). Differential detection of tumor cells using a combination of cell rolling, multivalent binding, and multiple antibodies. Analytical chemistry, 86(12), 6088–6094. https://doi.org/10.1021/ac501243a
-
Zhu, S., & Segura, T. (2014). HYDROGEL-BASED NANOCOMPOSITES OF THERAPEUTIC PROTEINS FOR TISSUE REPAIR. Current opinion in chemical engineering, 4, 128–136. https://doi.org/10.1016/j.coche.2013.12.009
-
Fierer, J. O., Veggiani, G., & Howarth, M. (2014). SpyLigase peptide-peptide ligation polymerizes affibodies to enhance magnetic cancer cell capture. Proceedings of the National Academy of Sciences of the United States of America, 111(13), E1176–E1181. https://doi.org/10.1073/pnas.1315776111
-
Song, Y., Huang, Y. Y., Liu, X., Zhang, X., Ferrari, M., & Qin, L. (2014). Point-of-care technologies for molecular diagnostics using a drop of blood. Trends in biotechnology, 32(3), 132–139. https://doi.org/10.1016/j.tibtech.2014.01.003
-
Wang, E. C., & Wang, A. Z. (2014). Nanoparticles and their applications in cell and molecular biology. Integrative biology : quantitative biosciences from nano to macro, 6(1), 9–26. https://doi.org/10.1039/c3ib40165k
-
Tassa, C., Liong, M., Hilderbrand, S., Sandler, J. E., Reiner, T., Keliher, E. J., Weissleder, R., & Shaw, S. Y. (2013). Microfluidic on-chip capture-cycloaddition reaction to reversibly immobilize small molecules or multi-component structures for biosensor applications. Journal of visualized experiments : JoVE, (79), e50772. https://doi.org/10.3791/50772
-
Sheng, W., Chen, T., Tan, W., & Fan, Z. H. (2013). Multivalent DNA nanospheres for enhanced capture of cancer cells in microfluidic devices. ACS nano, 7(8), 7067–7076. https://doi.org/10.1021/nn4023747
-
Lee, S., Yang, Y., Fishman, D., Banaszak Holl, M. M., & Hong, S. (2013). Epithelial-mesenchymal transition enhances nanoscale actin filament dynamics of ovarian cancer cells. The journal of physical chemistry. B, 117(31), 9233–9240. https://doi.org/10.1021/jp4055186
-
Cho, E. J., Holback, H., Liu, K. C., Abouelmagd, S. A., Park, J., & Yeo, Y. (2013). Nanoparticle characterization: state of the art, challenges, and emerging technologies. Molecular pharmaceutics, 10(6), 2093–2110. https://doi.org/10.1021/mp300697h
-
Jain, J., Veggiani, G., & Howarth, M. (2013). Cholesterol loading and ultrastable protein interactions determine the level of tumor marker required for optimal isolation of cancer cells. Cancer research, 73(7), 2310–2321. https://doi.org/10.1158/0008-5472.CAN-12-2956
-
Xiao, S., Turkyilmaz, S., & Smith, B. D. (2013). Convenient Synthesis of Multivalent Zinc(II)-Dipicolylamine Complexes for Molecular Recognition. Tetrahedron letters, 54(8), 861–864. https://doi.org/10.1016/j.tetlet.2012.11.103
-
Pearson, R. M., Patra, N., Hsu, H. J., Uddin, S., Král, P., & Hong, S. (2013). Positively Charged Dendron Micelles Display Negligible Cellular Interactions. ACS macro letters, 2(1), 77–81. https://doi.org/10.1021/mz300533w
-
Huang, W. Y., Davies, G. L., & Davis, J. J. (2013). High signal contrast gating with biomodified Gd doped mesoporous nanoparticles. Chemical communications (Cambridge, England), 49(1), 60–62. https://doi.org/10.1039/c2cc37545a
-
Tassa, C., Liong, M., Hilderbrand, S., Sandler, J. E., Reiner, T., Keliher, E. J., Weissleder, R., & Shaw, S. Y. (2012). On-chip bioorthogonal chemistry enables immobilization of in situ modified nanoparticles and small molecules for label-free monitoring of protein binding and reaction kinetics. Lab on a chip, 12(17), 3103–3110. https://doi.org/10.1039/c2lc40337d
-
Yang, Y., Sunoqrot, S., Stowell, C., Ji, J., Lee, C. W., Kim, J. W., Khan, S. A., & Hong, S. (2012). Effect of size, surface charge, and hydrophobicity of poly(amidoamine) dendrimers on their skin penetration. Biomacromolecules, 13(7), 2154–2162. https://doi.org/10.1021/bm300545b
-
Liu, J., Gray, W. D., Davis, M. E., & Luo, Y. (2012). Peptide- and saccharide-conjugated dendrimers for targeted drug delivery: a concise review. Interface focus, 2(3), 307–324. https://doi.org/10.1098/rsfs.2012.0009
-
Sunoqrot, S., Bae, J. W., Pearson, R. M., Shyu, K., Liu, Y., Kim, D. H., & Hong, S. (2012). Temporal control over cellular targeting through hybridization of folate-targeted dendrimers and PEG-PLA nanoparticles. Biomacromolecules, 13(4), 1223–1230. https://doi.org/10.1021/bm300316n