Recent Publications from the Tang Lab: 2018 – present
Chemists and Biologists work collaboratively on most current projects in the Tang group.
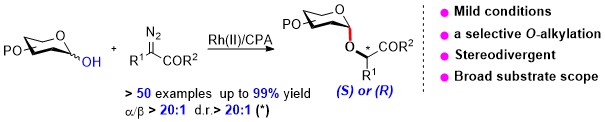
- “Rh(II) and Chiral Phosphoric Acid Co-catalyzed Selective O–H Insertions for Stereodivergent O-Alkylation of Glycosides” Wu, J.;‡ Jia, P.;‡ Tang, H.; Cai, D. and Tang, W.* J. Am. Chem. Soc. 2025, 147, 5871–5878. Link. (‡Equal Contribution)

- “Development of Prostate Cancer-Targeting BRD4 Intramolecular Bivalent Molecular Glue Degraders” Cai, D.;‡ Li, C.;‡ Bio Idrissou, M.; Hawkins, N. J.; Mudududdla, R.; Chen, X.; Nguyen, T. T.; Li, X.; Hernandez, R.; Tang, W.* ChemRxiv 2024. Link. (‡Equal Contribution)
- “Development of Potent and Selective RIPK1 Degraders with In Vivo Efficacy for Cancer Treatment” Zhang, Z.;‡ Li, C.;‡ Hawkins, N. J.;‡ Mudududdla, R.; Nie, Y.; Liu, P.-K.; Huang, P.; Del Rio, N. M.; Chang, H.; Brown, M. E.; Li, L.; Tang, W.* ChemRxiv 2024. Link. (‡Equal Contribution)
- “Development of Folate Receptor Targeting Chimeras for Cancer Selective Degradation of Extracellular Proteins” Zhou, Y.; Li, C.; Chen, X.; Zhao, Y.; Liao, Y.; Huang, P.; Wu, W.; Nieto, N. S.; Li, L.; and Tang, W.* Nat. Commun. 2024, 15, 8695. Link. Link to PDF file.

- “AI is a viable alternative to high throughput screening: a 318-target study” The Atomwise AIMS Program (>100 authors) Sci. Rep. 2024, 14, 7526. Link.
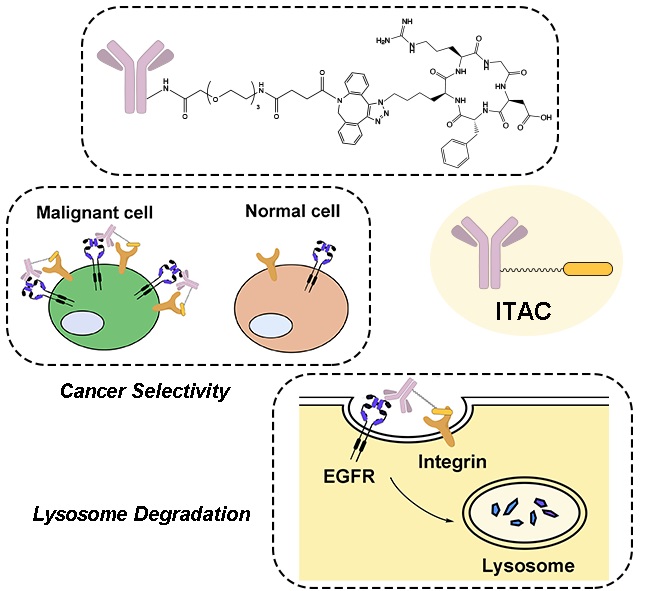
- “Development of Integrin Targeting Chimeras (ITACs) for the Lysosomal Degradation of Extracellular Proteins” Zhou, Y.; Liao, Y.; Zhao, Y.; Tang, W.* ChemMedChem 2024, 19, e202300643. Link.
- “BCR::ABL1 Proteolysis-targeting chimeras (PROTACs): The new frontier in the treatment of Ph+ leukemias?” Cruz-Rodriguez, N.; Tang, H.; Benjamin Bateman, B.; Tang, W.; Deininger, M.* Leukemia 2024, 38, 1885–1893. Link.
- “Non-Markovian Dynamic Models Identify Non-Canonical KRAS-VHL Encounter Complex Conformations for Novel PROTAC Design” Qiu, Y.; Wiewiora, R. P.; Izaguirre, J. A.; Xu, H.; Sherman, W.; Tang, W.*; Huang, X.* JACS Au 2024, 4, 3857-3868. Link.
- “Development of Phenyl-substituted Isoindolinone- and Benzimidazole-Type Cereblon Ligands for Targeted Protein Degradation” Nie, X.;‡ Zhao, Y.;‡ Tang, H.;‡ Zhang, Z.; Liao, J.; Almodóvar-Rivera, C. M.; Sundaresan, R.; Xie, H.; Guo, L.; Wang, B.; Guan, H.; Xing, Y.; and Tang, W.* ChemBioChem 2024, 25, e202300685. Link. (‡Equal Contribution)
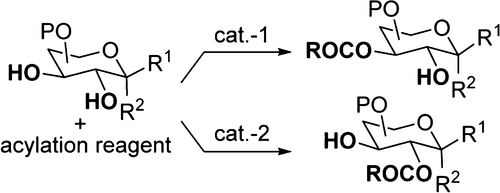
- “Stereo- and Site-selective Acylation in Carbohydrate Synthesis” ; Wen, P.; and Tang, W.* Synlett 2024, 35, 1745-1762. (an account for our work in the past 10 years or so) Link.
- “Targeted Degradation of Extracellular Secreted and Membrane Proteins” Chen, X.; Zhou, Y. ; Zhao, Y.; and Tang, W.* Trends. Pharmacol. Sci. 2023, 44, 762-775. Link.

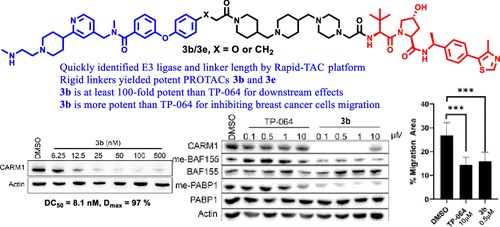
- “Development of Potent and Selective Coactivator-Associated Arginine Methyltransferase 1 (CARM1) Degraders” Xie, H.;‡ Bacbac, M. S.;‡ Ma, M.; Kim, E.-J.; Wang, Y.; Wu, W.; Li, L.; Xu, W.* and Tang, W.* J. Med. Chem. 2023, 66, 13028–13042. Link. (‡Equal Contribution).
- “Dynamic Kinetic Stereoselective Glycosylation via Rh(II) and Chiral Phosphoric Acid-Cocatalyzed Carbenoid Insertion to Anomeric OH Bond for the Synthesis of Glycoconjugates” Wu, J.;‡ Jia, P.;‡ Kuniyil, R.;‡ Liu, P.* and Tang, W.* Angew. Chem. In. Ed. 2023, 62, e202307144. Link. (‡Equal Contribution) Highlighted as “hot paper”
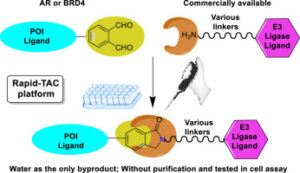
- “A Modular Chemistry Platform for the Development of a Cereblon E3 Ligase-based Partial PROTAC Library” Almodóvar-Rivera, C. M.; Zhang, Z.; Li, J.; Xie, H.; Zhao, Y.; Guo, L.; Mannhardt, M. G. and Tang, W.* ChemBioChem 2023, 24, e202300482. Link.
- “A platform for the rapid synthesis of molecular glues (Rapid-Glue) under miniaturized conditions for direct biological screening” Li, J.;‡ Li, C.;‡ Zhang, Z.;‡ Zhang, Z.;‡ Wu, Z.; Liao, J.; Wang, Z.; McReynolds, M.; Xie, H.; Guo, L.; Fan, Q.; Peng, J. and Tang, W.* Eur. J. Med. Chem. 2023, 258, 115567. Link. (‡Equal Contribution)

- “Development of Oligomeric Mannose-6-phosphonate Conjugates for Targeted Protein Degradation” Stevens, C. M.‡; Zhou, Y.‡; Teng, P.‡; Rault, L. N.; Liao, Y.; Tang, W.* ACS Med. Chem. Lett. 2023, 14, 719-726. Link. (‡Equal Contribution)

- “Development of Substituted Phenyl Dihydrouracil as the Novel Achiral Cereblon Ligands for Targeted Protein Degradation” Xie, H.‡; Li, C.‡; Tang, H.; Tandon, I.; Liao, J.; Roberts, B. L.; Zhao, Y.; Tang, W.* J. Med. Chem. 2023, 66, 2904-2917. Link. (‡Equal Contribution)

- “LPA81: Discovery of an Exceptionally Potent Protac Degrading Native and Mutant BCR-ABL1 Oncoprotein in CML” Milad Rouhimoghadam, M.; Tang, H.; Liao, J.; Bates, B.; Uribe-Cano, D.; Zhao, H.; Tang, W.; Deininger, M. W. Blood 2022, 140 (Supplement 1), 485–486. Link.
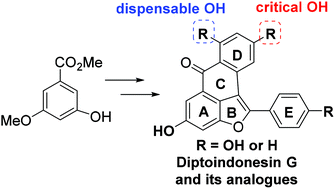
- “Diptoindonesin G is a middle domain HSP90 modulator for cancer treatment” Donahue, K.‡; Xie, H.‡; Li, M.; Gao, A.; Ma, M.; Wang, Y.; Tipton, R.; Semanik, N.; Primeau, T.; Li, S.; Li, L.; Tang, W.*; Xu, W.* J. Biol. Chem. 2022, 298 , 102700. Link. (‡Equal Contribution) (Editor’s pick)
- “Development of Selective FGFR1 Degraders using a Rapid Synthesis of Proteolysis Targeting Chimera (Rapid-TAC) Platform” Guo, L.‡; Liu, J.‡; Nie, X.‡; Wang, T.; Ma, Z.- X., Yin, D. Tang, W.* Bioorg. Med. Chem. Lett. 2022, 75, 128982. Link. (‡Equal Contribution)

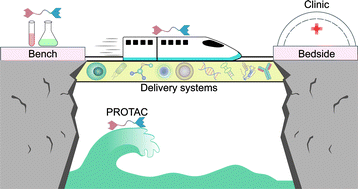
- “Proteolysis-targeting chimera (PROTAC) delivery system: advancing protein degraders towards clinical translation” Chen, Y.; Tandon, I.; Heelan, W.; Wang, Y.; Tang, W.* and Hu, Q.* Chem. Soc. Rev. 2022, 51, 5330-5350. Link.
- “A Platform for the Rapid Synthesis of Proteolysis Targeting Chimeras (Rapid-TAC) under Miniaturized Conditions” Guo, L.‡; Zhou, Y.‡; Nie, X.‡; Zhang, Z.; Zhang, Z.; Li, C.; Wang, T.; and Tang, W.* Eur. J. Med. Chem. 2022, 236, 114317. Link. (‡Equal Contribution)
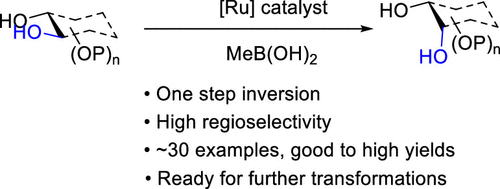
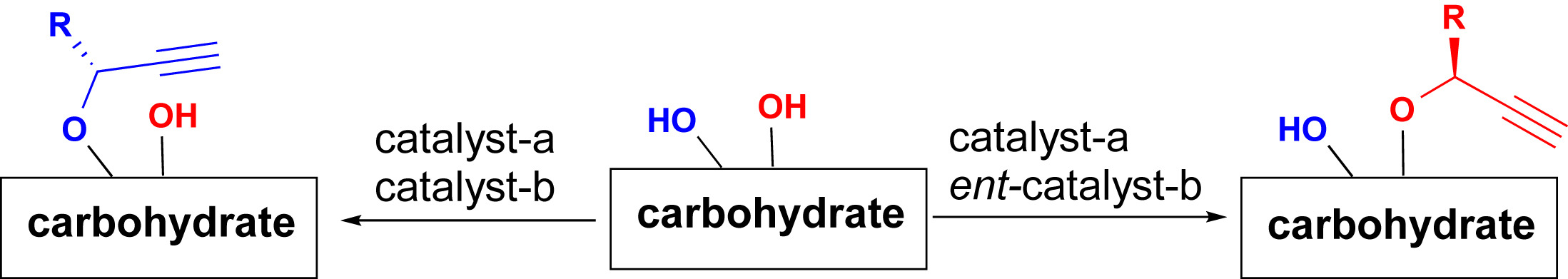
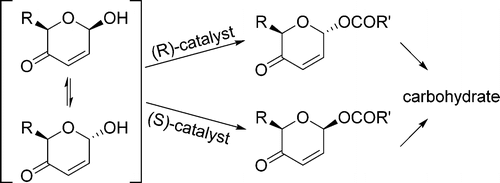
- “A General Strategy for the Synthesis of Rare Sugars via Ru(II)-catalyzed and Boron-mediated Selective Epimerization of 1,2-trans-diols to 1,2-cis-diols” Li, X.; Wu, J.; and Tang, W.* J. Am. Chem. Soc. 2022, 144, 3727-3736. Link. Highlighted in Organic Chemistry Highlight – Link.
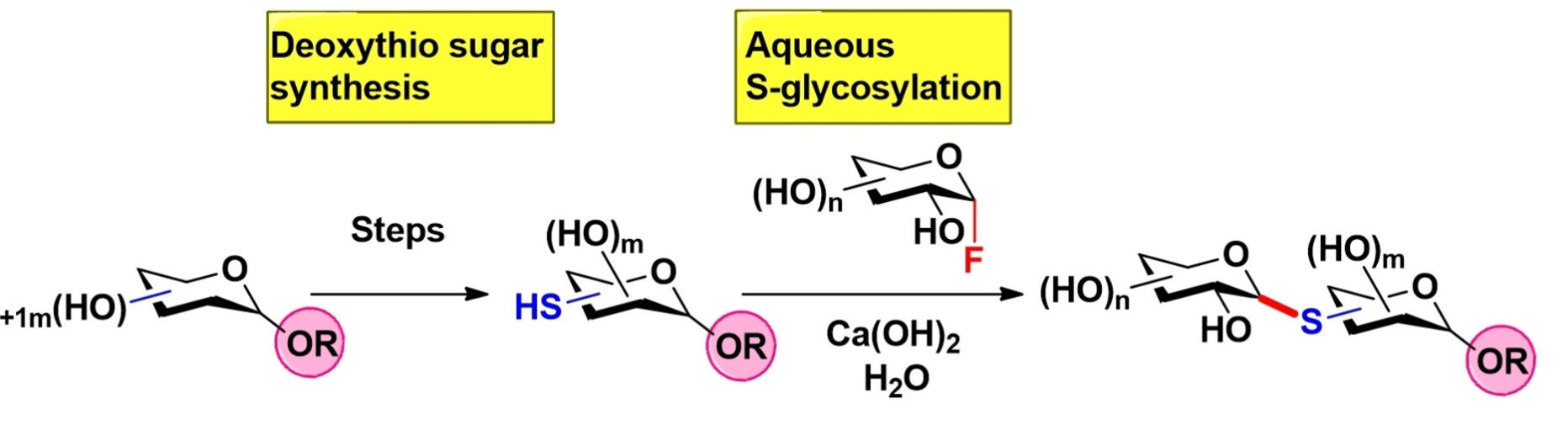
- “Streamlined Iterative Assembly of Thio-oligosaccharides by Aqueous S-Glycosylation of Diverse Deoxythio Sugars” Wen, P.‡; Jia, P.‡; Fan, Q.; McCarty, B. J. and Tang, W.* ChemSusChem 2022, 15, e202102483. Link. (‡Equal Contribution)
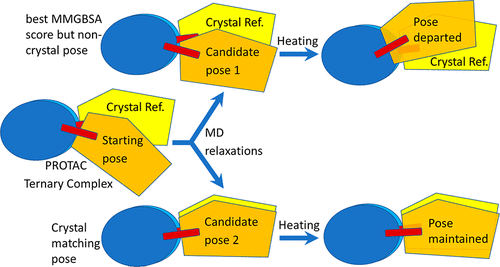
- “In Silico Modeling and Scoring of PROTAC-Mediated Ternary Complex Poses” Liao, J.; Nie, X.; Unarta, I. C.; Ericksen, S. S.*; and Tang, W.* J. Med. Chem. 2022, 65, 6116-6132. Link.
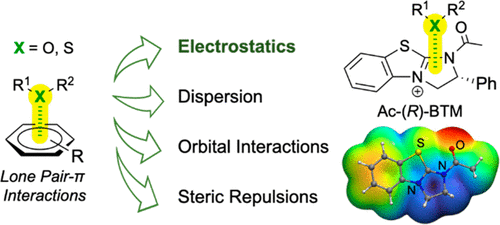
- “Energy Decomposition Analysis Reveals the Nature of Lone Pair−π Interactions with Cationic π Systems in Catalytic Acyl Transfer Reactions” Hao, H.; Qi, X.; Tang, W.* and Liu, P.* Org. Lett. 2021, 23, 4411–4414. Link.
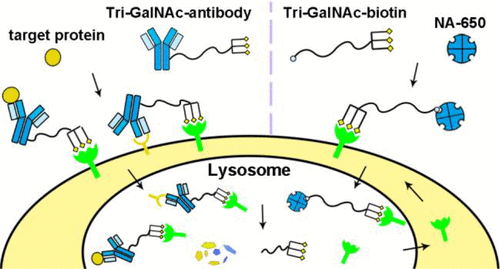
- “Development of Triantennary N-Acetylgalactosamine Conjugates as Degraders for Extracellular Proteins” Zhou, Y.; Teng, P.; Montgomery, N. T.; Li, X.; and Tang, W.* ACS Cent. Sci, 2021, 7, 499-506. Link.
- “Evaluation of the binding affinity of E3 ubiquitin ligase ligands by cellular target engagement and in-cell ELISA assay” Yang, K.; Zhou, Y.; Roberts, R. L.; Nie, X.; Tang, W.* Star Protocols 2021, 2, 100288. Link.
- “A neuroanatomical mechanism linking perinatal TCDD exposure to lower urinary tract dysfunction in adulthood” Turco, A. E.; Oakes, S. R.; Stietz, K. P. K. Dunham, C. L.; Joseph, D. B.; Chathurvedula, T. S.; Girardi, N. M.; Schneider, A. J.; Gawdzik, J.; Sheftel, C. M.; Wang, P.; Wang, Z.; Bjorling, D. E.; Ricke, W. A.; Tang, W.; Hernandez, L. L.; Keast, J. R.; Bonev, A. D.; Grimes, M. D.; Strand, D. W.; Tykocki, N. R.; Tanguay, R. L.; Peterson, R. E.; Vezina, C. M. Dis. Models Mech. 2021, 14, dmm049068. Link.
- “Development of MDM2 Degraders Based on Ligands Derived from Ugi Reactions:
Lessons and Discoveries” Wang, B.‡; Liu, J.‡; Tandon, I.; Wu, S.; Teng, P.; Liao, J.; and Tang, W.* Eur. J. Med. Chem. 2021, 219, 113425. Link. (‡Equal Contribution)
- “A dancing nickel in asymmetric catalysis: Enantioselective synthesis of boronic esters by 1,1-addition to terminal alkenes” McCarty, B. J. and Tang, W.* Green Syn. Cat. 2021, 2, 1-3. Link.
- “Transition Metal-Catalyzed Selective Carbon−Carbon Bond Cleavage of Vinylcyclopropanes in Cycloaddition Reactions” Wang, J.; Blaszczyk, S. A.; Li, X.;* and Tang, W.* Chem. Rev. 2021, 121, 110-139. Link.
- “A marine microbiome antifungal targets urgent-threat drug-resistant fungi” , T. S. Science, 2020, 370 (issue 6519), 974-978. Link.
- “From Methylene Bridged Diindole to Carbonyl Linked Benzimidazoleindole: Development of Potent and Metabolically Stable PCSK9 Modulators” Xie, H.;‡ Yang, K.;‡ Winston-McPherson, G. N.; Stapleton, D. S.; Keller, M. P.; Attie, A. D.; Smith, K. A.; and Tang, W.* Eur. J. Med. Chem. 2020, 206, 112678-112692. Link. (‡Equal Contribution)
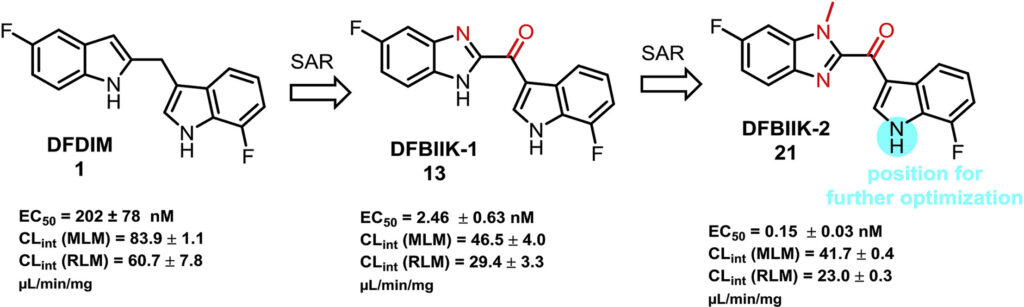
- “Mild Cu(OTf)2-mediated C-glycosylation with Chelation-Assisted Picolinate as a Leaving Group” Ye, W.;‡ Stevens, C. M.;‡ Wen, P.;‡ Simmons, C. J.; and Tang, W.* J. Org. Chem. 2020, 85, 16218–16225. Link. (‡Equal Contribution) (Special Issue on A New Era of Discovery in Carbohydrate Chemistry)
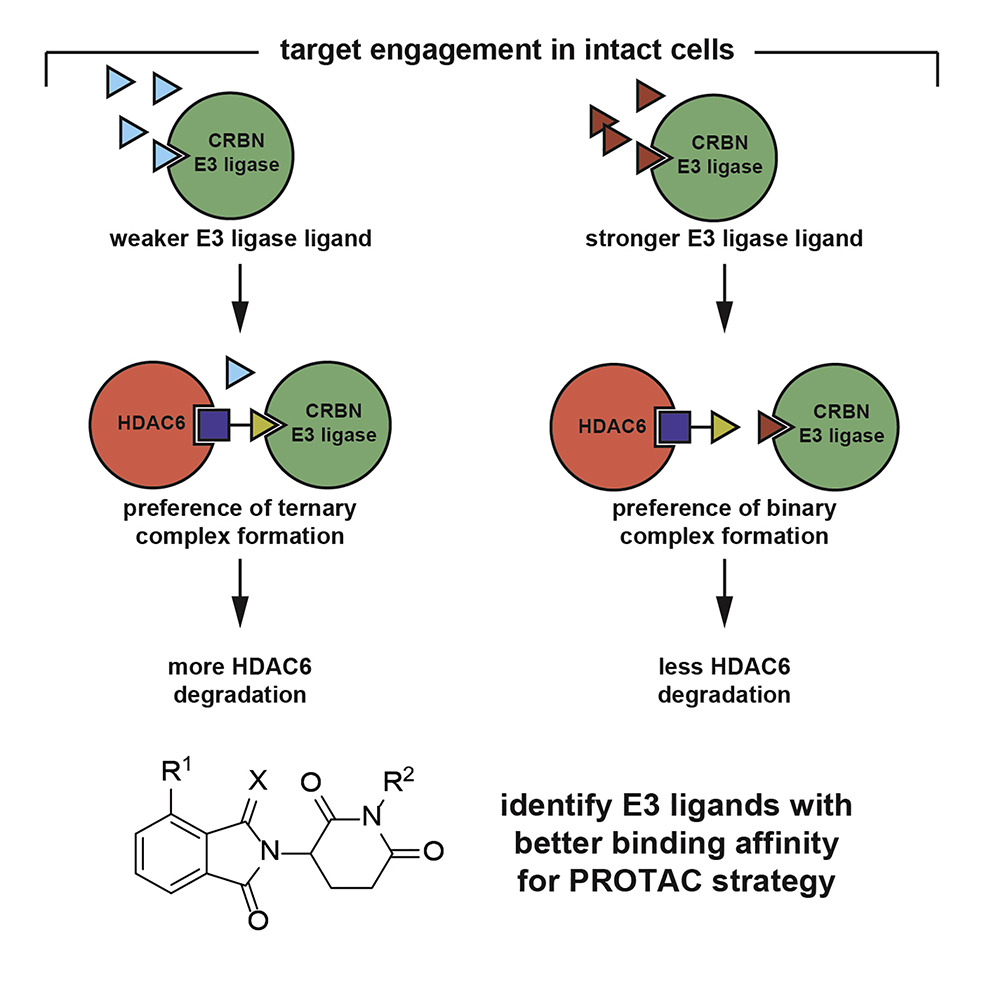
- “A Cell-based Target Engagement Assay for the Identification of Cereblon E3 Ubiquitin Ligase Ligands and Their Application in HDAC6 Degraders” Yang, K.;‡ Zhao, Y.;‡ Nie, X.; Wu, H.; Wang, B.; Almodovar-Rivera, C. M.; Xie, H.;* Tang, W.* Cell Chem. Biol. 2020, 27, 866-876. Link. (‡Equal Contribution
- “Two-stage Strategy for Development of Proteolysis Targeting Chimeras and its Application for Estrogen Receptor Degraders” Roberts, B. L.;‡ Ma, Z.-X.;‡ Gao, A.; Leisten, E. D.; Yin, D.; Xu, W.; and Tang, W.* ACS Chem. Biol. 2020, 15, 1487–1496. Link. (‡Equal Contribution)
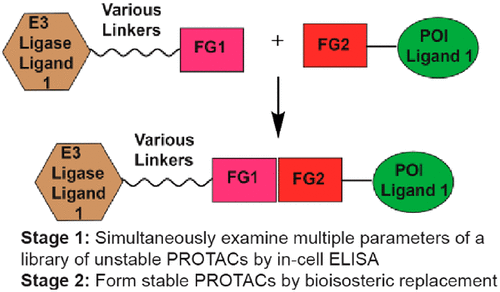
- “Chemical Synthesis and Biological Application of Modified Oligonucleotides” Glazier, D. A.;‡ Liao, J.;‡ Roberts, B. L.;‡ Li, X.; Yang, K.; Stevens, C. M.; and Tang, W.* Bioconjugate Chem. 2020, 31, 1213-1233. Link. (‡Equal Contribution)
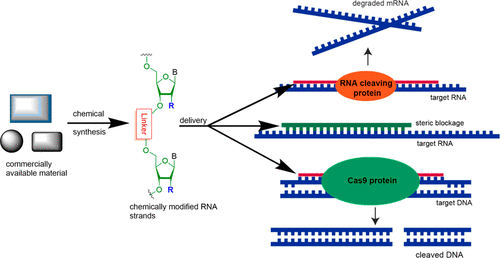
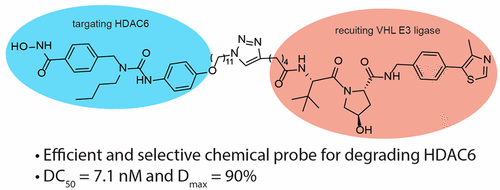
- “Development of Selective Histone Deacetylase 6 (HDAC6) Degraders Recruiting Von Hippel–Lindau (VHL) E3 Ubiquitin Ligase” Yang, K.;‡ Wu, H.;‡ Zhang, Z.; Leisten, E. D.; Nie, X.; Liu, B.; Wen, Z.; Zhang, J.; Cunningham, M. D. and Tang, W.* ACS Med. Chem. Lett. 2020, 11, 575-581. Link. (‡Equal Contribution)
- “Synthesis of Glycosyl Chlorides and Bromides by Chelation Assisted Activation of Picolinic Esters under Mild Neutral Conditions” Wen, P.;‡ Simmons, C. J.;‡ Ma, Z.-X.; Blaszczyk, S. A.; Balzer, P. G.; Ye, W.; Duan, X.; Wang, H.-Y.; Yin, D.; Stevens, C. M.; and Tang, W.* Org. Lett. 2020, 22, 1495-1498. Link. (‡Equal Contribution)
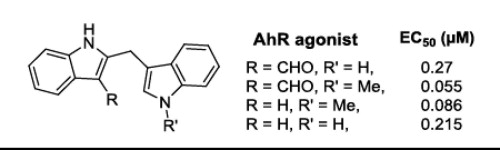
- “Synthesis and Biological Evaluation of FICZ Analogues as Agonists of Aryl Hydrocarbon Receptor” Wu, H.;‡ Liu, B.;‡ Yang, K.;‡ Winston-McPherson, G. N.; Leisten, E. D.; Vezina, C. M.; Ricke, W. A.; Peterson, R. E.; and Tang, W.* Bioorg. Med. Chem. Lett. 2020, 30, 126959. Link. (‡Equal Contribution)
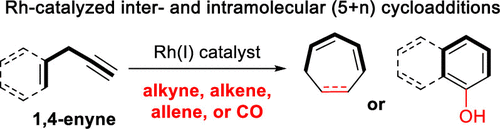
- “Rhodium-Catalyzed (5 + 2) and (5 + 1) Cycloadditions Using 1,4-Enynes as Five-Carbon Building Blocks” Blaszczyk, S. A.; Glazier, D. A.; and Tang, W.* Acc. Chem. Res. 2020, 53, 231-243. Link.
- “Mechanism of Activation for the Sirtuin 6 Protein Deacylase” Klein, M. A., Liu, C.; Kuznetsov, V. I.; Feltenberger, J. B.; Tang, W.; Denu, J. M.* J. Biol. Chem. 2020, 295, 1385-1399. Link.
- “In utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure exacerbates urinary dysfunction in hormone-treated C57BL/6J mice through a non-malignant mechanism involving proteomic changes in the prostate that differ from those elicited by testosterone and estradiol” Turco, A. E.; Thomas, S; Crawford, L. K.; Tang, W.; Peterson, R. E.; Li, L.; Ricke, W. A.; Vezina, C. M.* Am. J. Clin. Exp. Urol. 2020, 8, 59. Link.
- “Tissue-specific quantification and localization of androgen and estrogen receptors in prostate cancer” Sehgal, P. D.; Bauman, T. M.; Nicholson, T. M.; Vellky, J. E.; Ricke, E. A.; Tang, W.; Xu, W.; Huang, W.; Ricke, W. A.* Hum. Pathol. 2019, 89, 99-108. Link.
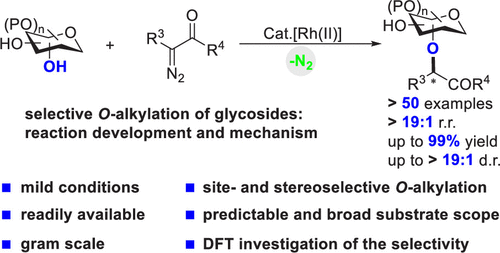
- “Site- and Stereoselective O-Alkylation of Glycosides by Rh(II)-Catalyzed Carbenoid Insertion” Wu, J.;‡ Li, X.;‡ Qi, X.; Duan, X.; Cracraft, W. L.; Guizei, I. A.; Liu, P.;* and Tang, W.* J. Am. Chem. Soc. 2019, 141, 19902-19910. Link. (‡Equal Contribution)
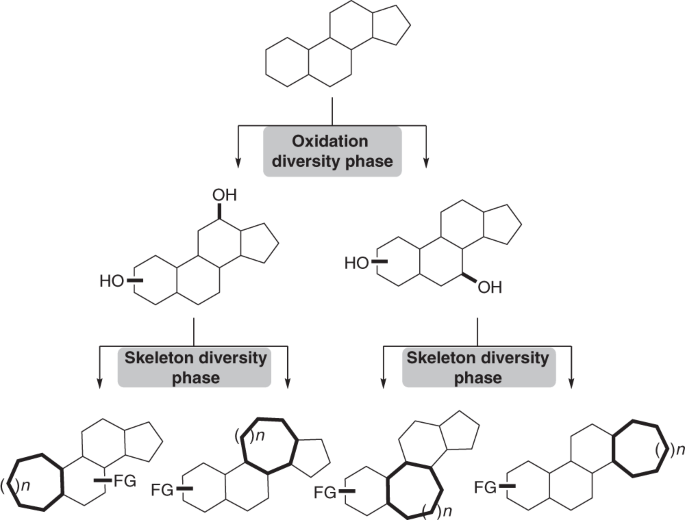
- “A general strategy for diversifying complex natural products to polycyclic scaffolds with medium-sized rings” Zhao, C.; Ye, Z.; Ma, Z.-X.; Wildman, S. A.; Blaszczyk, S. A.; Hu, L.; Guizei, I. A.; Tang, W.* Nat. Commun. 2019, 10, 4015. Link.
- “Discovery of 2,3′-diindolylmethanes as a novel class of PCSK9 modulators” Winston-McPherson, G. N.;‡ Xie, H.;‡ Yang, K.;‡ Li, X.; Shu, D.; Tang, W.* Bioorg. Med. Chem. Lett. 2019, 29, 2345-2348. Link. (‡Equal Contribution)
- “Development of Multi-Functional Histone Deacetylase 6 Degraders with Potent Anti-Myeloma Activity” Wu, H.; ‡ Yang, K.; ‡ Zhang, Z.; Leisten, E. D.; Li, Z.; Xie, H.; Liu, J.; Smith, K. A.; Novakova, Z.; Barinka, C.; and Tang, W.* J. Med. Chem. 2019, 62, 7042-7057. Link. (‡Equal Contribution)
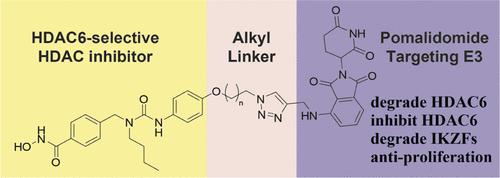
- “Site‐ and Stereoselective Phosphoramidation of Carbohydrates Using a Chiral Catalyst and a Chiral Electrophile” Glazier, D. A.; Schroeder, J. M.; Blaszczyk, S. A.; Tang, W.* Adv. Syn. Cat. 2019, 361, 3729-3732. Link.
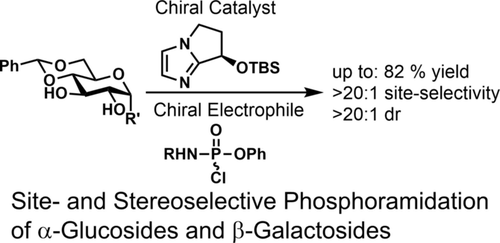
- “Development of selective small molecule MDM2 degraders based on nutlin” Wang, B.;# Wu, S.;# Liu, J.; Yang, K.; Xie, H.; and Tang, W.* Eur. J. Med. Chem. 2019, 176, 476-491. Link.(#Equal Contribution)
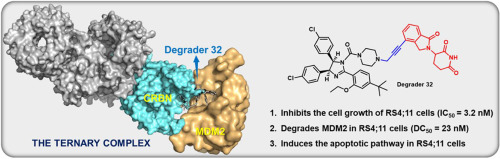
- “S‐Adamantyl Group Directed Site‐Selective Acylation and Its Applications in the Streamlined Assembly of Oligosaccharides” Blaszczyk. S. A.;# Xiao, G.;# Wen, P.;# Hao, H.; Wu, J.; Wang, B.; Carattino, F.; Li, Z.; Glazier, D. A.; McCarty, B. J.; Liu, P.* and Tang, W.* Angew. Chem. Int. Ed. 2019, 58, 9542-9546. Link. (#Equal Contribution)
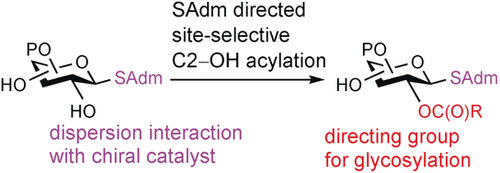
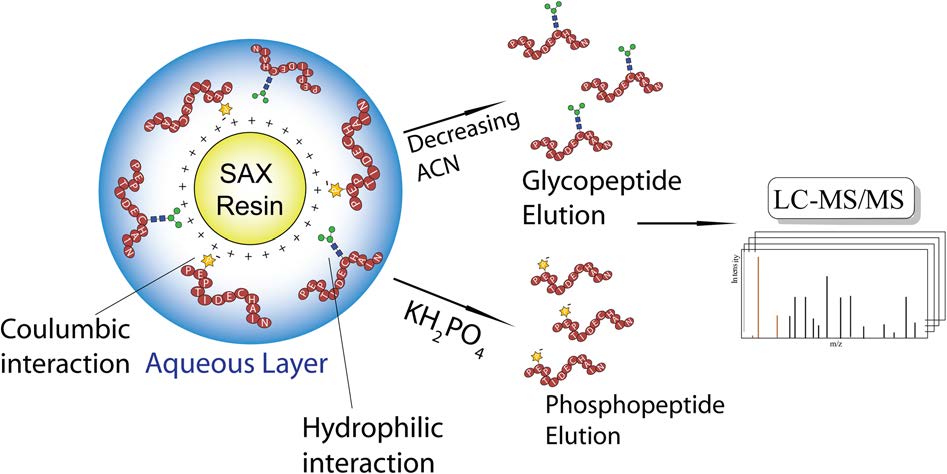
- “Finding the Sweet Spot in SAX-ERLIC Mobile Phase for Simultaneous Enrichment of N-glyco and Phospho- peptides” Cui, Y.; Yang, K.; Tabang D. N.; Huang, J.; Tang, W., Li, L.* J. Am. Soc. Mass Spectrom. 2019, 30, 2491–2501. Link.
- “Identification of a novel class of RIP1/RIP3 dual inhibitors that impede cell death and inflammation in mouse abdominal aortic aneurysm models” Zhou, T.; Wang, Q.; Phan, N.; Ren, J.; Yang, H.; Feldman, C. C.; Feltenberger, J. B.; Ye, Z.; Wildman, S. A.; Tang, W., Liu, B.* Cell Death & Disease 2019, 10, 226. Link.
- “Intermolecular Regio- and Stereoselective Hetero-[5+2] Cycloaddition of Oxidopyrylium Ylides and Cyclic Imines” Zhao, C.; Glazier, D. A.; Yang, D.; Yin, D.; Guzei, I. A.; Aristov, M. M.; Liu, P.* and Tang, W.* Angew. Chem. Int. Ed. 2019, 58, 887-891. Link.
![Graphical abstract from Tang study "Intermolecular Regio- and Stereoselective Hetero-[5+2] Cycloaddition of Oxidopyrylium Ylides and Cyclic Imines"](https://pharmacy.wisc.edu/faculty/tang-research-group/wp-content/uploads/sites/10/2024/09/anie201811896-toc-0001-m-300x79-1.jpg)
- “Recent advances in site-selective functionalization of carbohydrates mediated by organocatalysts” Blaszczyk, S. A.; Homan, T. C.; Tang, W.* Carbohydr. Res. 2019, 471, 64-77. Link.
- “Organocatalyst-Mediated Dynamic Kinetic Enantioselective Acylation of 2-Chromanols” Glazier, D. A.; Schroeder, J. M.; Liu, J.; Tang, W.* Adv. Syn. Cat. 2018, 360, 4646-4649. Link.
- “Development of the first small molecule histone deacetylase 6 (HDAC6) degraders.” Yang, K.; Song, Y.; Xie, H.; Wu, H.; Wu, Y.-T.; Leisten, E. D.; Tang W.* Bioorg. Med. Chem. Lett. 2018, 28, 2493-2497. Link.
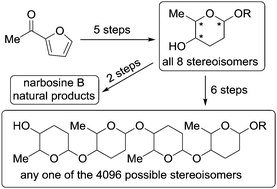
- “Catalytic Asymmetric Synthesis of All Possible Stereoisomers of 2,3,4,6‐Tetradeoxy‐4‐Aminohexopyranosides” Zhu Z.; Glazier, D. A.; Yang D.; Tang, W.* Adv. Syn. Cat. 2018, 360, 2211-2215. Link.

- “Trace derivatives of kynurenine potently activate the aryl hydrocarbon receptor (AHR)” Seok, S.-H.; Ma, Z.-X.; Feltenberger, J. B.; Chen, H.; Chen, H.; Scarlett, C.; Lin, Z.; Satyshur, K. A.; Cortopassi, M.; Jefcoate, C. R.; Ge, Y.; Tang, W.; Bradfield, C. A.; and Xing, Y.* J. Biol. Chem. 2018, 293, 1994-2005. Link.
- “Iridium-Catalyzed Dynamic Kinetic Stereoselective Allylic Etherification of Achmatowicz Rearrangement Products.” Zhu, Z.‡; Wang, H.-Y.‡; Simmons, C. J.; Tseng, P.-S.; Qiu, X.; Zhang, Y.; Duan, X.; Yang, J.-K.; and Tang W.* Adv. Syn. Cat. 2018, 360, 595-599. (‡Equal Contribution) Link.
- “Chiral Reagents in Glycosylation and Modification of Carbohydrates.” Wang, H.-Y.; Blaszczyk, S. A.; Xiao, G.; and Tang W.* Chem. Soc. Rev. 2018, 47, 681-701. Link.
-
Publications: 2013-2017
- “AB3-loaded and tumor-targeted unimolecular micelles for medullary thyroid cancer treatment.” Jaskula-Sztul, R.; Chen, G.; Dammalapati, A.; Harrison, A.; Tang, W.; Gong, S.;* and Chen, H.* J. Mater. Chem. 2017, 5, 151-159. Link.
- “Addressing the Challenge of Carbohydrate Site Selectivity by Synergistic Catalysis.”
Blaszczyk, S. A. and Tang W.* Chem 2017, 3, 722–723. Link.
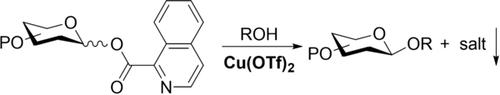
- “Isoquinoline-1-carboxylate as a Traceless Leaving Group for Chelation-Assisted Glycosylation under Mild Neutral Conditions.” Wang, H.-Y.; Simmons, C. J.; Blaszczyk, S. A.; Balzer, P. G.; Luo, R.; Duan, X.; and Tang W.* Angew. Chem. Int. Ed. 2017, 56, 15698–15702 . Link. SoP News.
- “Harnessing the Reactivity of Iridium Hydrides by Air: Iridium- Catalyzed Oxidation of Aldehydes to Acids in Water.” Yang, Z.; Luo, R.; Zhu, Z.; Yang, X.; and Tang W.* Organometallics. 2017, 36, 4095–4098. Link.
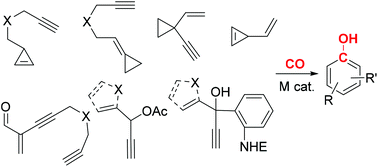
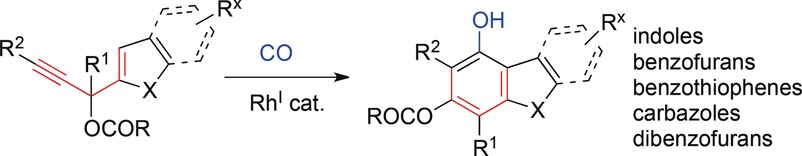
- “Transition metal mediated carbonylative benzannulations.” Song, W.; Blaszczyk, S. A.; Liu, J.; Wang, S.;* and Tang W.* Org. Biomol. Chem. 2017, 15, 7490-7504. Link.
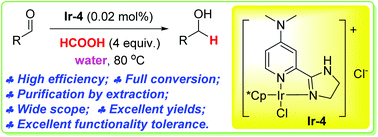
- “Iridium-catalyzed highly efficient chemoselective reduction of aldehydes in water using formic acid as the hydrogen source.” Yang, Z.; Zhu, Z.; Luo, R.; Qiu, X.; Liu, J.-L.; Yang, J.-K.; and Tang W.* Green Chem. 2017, 19, 3296-3301. Link.
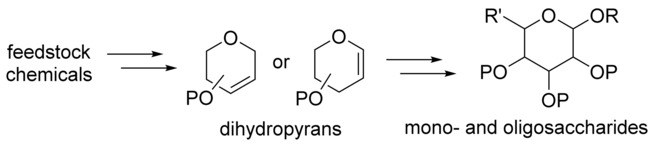
- “De novo Synthesis of Mono- and Oligosaccharides via Dihydropyran Intermediates.”
Song, W.; Wang, S.* and Tang, W.* Chem. Asian J. 2017, 12, 1027-1042. Link.
- “Catalytic Site-Selective Acylation of Carbohydrates Directed by Cation–n Interaction.”
Xiao, G.; Cintron-Rosado, G. A.; Glazier, D. A.; Xi, B.-m.; Liu, C.; Liu, P.* and Tang, W.* J. Am. Chem. Soc. 2017, 139, 4346-4349. Link. SoP News.
- “Neuroendocrine Tumor-Targeted Upconversion Nanoparticle-Based Micelles for Simultaneous NIR-Controlled Combination Chemotherapy and Photodynamic Therapy, and Fluorescence Imaging.” Chen, G.; Jaskula-Sztul, R.; Esquibel, C. R.; Lou, I.; Zheng, Q.; Dammalapati, A.; Harrison, A.; Eliceiri, K. W.; Tang, W.; Chen, H.;* Gong, S.* Adv. Funct. Mater. 2017, 27, 1604671. Link.
- “Chiral Catalyst-Directed Dynamic Kinetic Diastereoselective Acylation of Anomeric Hydroxyl Groups and a Controlled Reduction of the Glycosyl Ester Products.”
Wang, H.-Y.; Simmons, C. J.; Zhang, Y.; Smits, A. M.; Balzer, P. G.; Wang, S.;* and Tang, W.* Org. Lett. 2017, 19, 508-511. Link.
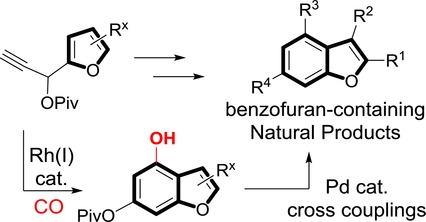
- “Synthesis of Highly Substituted Benzofuran-containing Natural Products via Rh-catalyzed Carbonylative Benzannulation.” Liu, J.-t.; Simmons, C. J.; Xie, H.; Yang, F.; Zhao, X-l.;* Tang, Y.;* and Tang, W.* Adv. Syn. Catal. 2017, 359, 693-697. link. Highlight in Synfacts, 2017, 13(05): 0478 (DOI: 10.1055/s-0036-1590363).
- “Discovery of selective small-molecule HDAC6 inhibitor for overcoming proteasome inhibitor resistance in multiple myeloma.” Hideshima, T.; Qi, J.; Paranal, R. M.; Tang, W.; Greenberg, E.; West, N.; Colling, M. E.; Estiu, G.; Mazitschek, R.; Perry, J. A.; Ohguchi, H.; Cottini, F.; Mimura, N.; Görgün, G.; Tai, Y.-T.; Richardson, P. G.; Carrasco, R. D.; Wiest, O.; Schreiber, S. L.; Anderson, K. C.;* Bradner, J. E.* Proc. Natl. Acad. Sci. U.S.A. 2016, 113, 13162-13167. Link.
- “Author Profile ” Tang, W. Angew. Chem. Int. Ed. 2016, 55, 12142. Link.
- “Total Synthesis of Diptoindonesin G and Its Analogues as Selective Modulators of Estrogen Receptors.” Liu, J.-t.; Do, T. J.; Simmons, C. J.; Lynch, J. C.; Gu, W.; Ma, Z.-X.; Xu, W.; and Tang, W.* Org. Biomol. Chem. 2016, 14, 8927-8930. Link.
- “Rhodium(I)-Catalyzed Benzannulation of Heteroaryl Propargylic Esters: Synthesis of Indoles and Related Heterocycles.” Li, X.; Xie, H.; Fu, X.; Liu, J.-t.; Wang, H.-y.; Xi, B.-m.;* Liu, P.;* Xu, X.;* and Tang, W.* Chem. Eur. J. 2016, 22, 10410-10414. Link.
- “Rhodium-catalyzed [5+2] Cycloaddition of 3-Acyloxy-1,4-enyne and Alkene or Allene.”
Song, W.; Lynch, J. C.; Shu, X.-z.; and Tang, W.* Adv. Syn. Catal. 2016, 358, 2007-2011. Link.
![Graphical abstract image for "Rhodium-catalyzed [5+2] Cycloaddition of 3-Acyloxy-1,4-enyne and Alkene or Allene"](https://pharmacy.wisc.edu/faculty/tang-research-group/wp-content/uploads/sites/10/2024/09/61.jpg)
- “Rhodium-Catalyzed [5+2] Cycloaddition of Inverted 3-Acyloxy-1,4-enyne and Alkyne: Experimental and Theoretical Studies.” Li, X.; Song, W.; Zhao, X.-l.; Ke, X.; Xu, X.;* Liu, P.; Houk, K. N. and Tang, W.* Chem. Eur. J. 2016, 22, 7079-7083. Link.
![Graphical abstract image for Tang study "Rhodium-Catalyzed [5+2] Cycloaddition of Inverted 3-Acyloxy-1,4-enyne and Alkyne: Experimental and Theoretical Studies"](https://pharmacy.wisc.edu/faculty/tang-research-group/wp-content/uploads/sites/10/2024/09/chem201601195-toc-0001-m.png)
- “Synthesis of Carbazoles and Carbazole-Containing Heterocycles via Rhodium-Catalyzed Tandem Carbonylative Benzannulations.” Song, W.; Li, X.; Yang, K.; Zhao, X.-l.; Glazier, D. A.; Xi, B.-m.;* Tang, W.* J. Org. Chem. 2016, 81, 2930–2942. Link.
- “Mechanism and reactivity of rhodium-catalyzed intermolecular [5+1] cycloaddition of 3-acyloxy-1,4-enyne (ACE) and CO: A computational study.” Ke, X.-N.; Schienebeck, C. M.; Zhou, C.-C.; Xu, X.-F.;* Tang, W.* Chin. Chem. Lett. 2015, 26, 730-734. Link.
- “Chiral Catalyst-Directed Dynamic Kinetic Diastereoselective Acylation of Lactols for De Novo Synthesis of Carbohydrate.” Wang, H.-Y.; Yang, K.; Yin, D.; Liu, C.; Glazier, D. A.; Tang, W.* Org. Lett. 2015, 17, 5272-5275. Link.
- “Divergent De Novo Synthesis of All Eight Stereoisomers of 2,3,6-Trideoxyhexopyranosides and Their Oligomers.” Song, W.; Zhao, Y.;* Lynch, J. C.; Kim, H.; Tang, W.* Chem Commun. 2015, 51, 17475-17478. Link.
- “Rhodium-Catalyzed Stereoselective Intramolecular [5 + 2] Cycloaddition of 3-Acyloxy 1,4-Enyne and Alkene.”
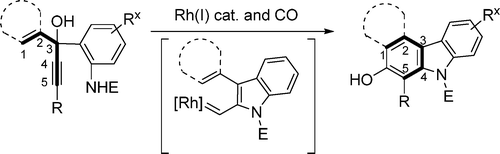
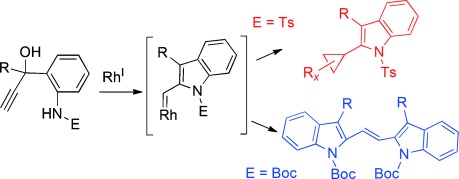
Shu, X.-Z.; Schienebeck, C. M.; Li, X.; Zhou, X.; Song, W.; Chen, L.; Guzei, I. A.; Tang, W.* Org. Lett. 2015, 17, 5128-5131. Link. - “Divergent Reactivity of Rhodium(I) Carbenes Derived from Indole Annulations.”
Li, X.; Li, H.; Song, W.; Tseng, P.-S.; Liu, L.-Y.*; Guzei, I. A.; Tang, W.* Angew. Chem. Int. Ed. 2015, 54, 12905-12908. Link.
- “Iridium-Catalyzed Dynamic Kinetic Isomerization: Expedient Synthesis of Carbohydrates from Achmatowicz Rearrangement Products.” Wang, H.-Y.; Yang, K.; Bennett S. R.; Guo, S.-R.;* Tang, W.* Angew. Chem. Int. Ed. 2015, 54, 8756–8759. Link.
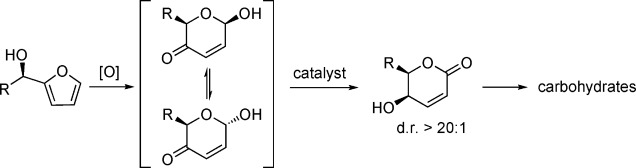
- “Novel Analogs Targeting Histone Deacetylase Suppress Aggressive Thyroid Cancer Cell Growth and Induce Re-differentiation.” Jang, S.; Yu, X. M.; Odorico, S.; Clark, M.; Jaskula-Sztul, R.; Schienebeck, C. M.; Kupcho, K. R.; Harrison, A. D.; Winston-McPherson, G. N.; Tang, W.; Chen, H.* Cancer Gene Ther. 2015, 22, 410. Link.
- “Synthesis of Substituted Tropones by Sequential Rh-Catalyzed [5+2] Cycloaddition and Elimination.” Song, W.; Xi, B.-m.;* Yang, K.; Tang, W.* Tetrahedron 2015, 71, 5979-5984. (Invited contribution for Prof. Barry Trost’s Tetrahedron Award.). Link.
![Graphical abstract image for Tang article "Synthesis of Substituted Tropones by Sequential Rh-Catalyzed [5+2] Cycloaddition and Elimination"](https://pharmacy.wisc.edu/faculty/tang-research-group/wp-content/uploads/sites/10/2024/09/1-s2.0-S0040402015005372-fx1.jpg)
- “Rhodium-Catalyzed Intermolecular [5+1] and [5+2] Cycloadditions Using 1,4-Enynes with an Electron-Donating Ester on the 3-Position.” Schienebeck, C. M.; Song, W.; Smits, A. M.; Tang, W.* Synthesis 2015, 47, 1076-1084. (invited feature article). Link.
- “Tumor Suppressor Role of Notch3 in Medullary Thyroid Carcinoma Revealed by Genetic and Pharmacological Induction.” Jaskula-Sztul, R.; Eide, J.; Tesfazghi, S.; Dammalapati, A.; Harrison, A. D.; Yu, X.-M.; Scheinebeck, C.; Winston-McPherson, G.; Kupcho, K. R.; Robers, M. B.; Hundal, A. K.; Tang, W.;* Chen, H.* Mol. Cancer Therap. 2015, 14, 499. Link.
- “Gold versus Rhodium: Divergent Reactivity Enabled by the Catalyst.” Winston-McPherson, G. N.; Tang, W.* ChemCatChem 2015, 7, 574-576. Link.
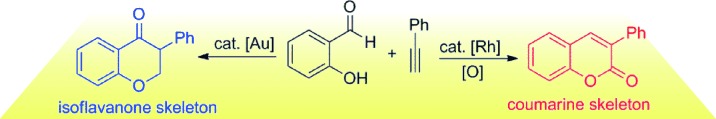
- “Copper-catalyzed tandem annulation/arylation for the synthesis of diindolylmethanes from propargylic alcohols.” Li, H.; Li, X.; Wang, H.-Y.; Winston-McPherson, G. N.; Geng, H.-M. J.; Guzei, I. A.; Tang, W.* Chem. Commun. 2014, 50, 12293-12296. Link.
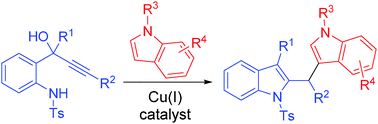
- “Synthesis of naturally occurring tropones and tropolones.” Liu, N.; Song, W.; Schienebeck, C. M.; Zhang, M.* Tang, W.* Tetrahedron. 2014, 70, 9281-9305. (Invited review) Link.
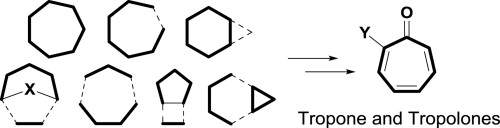
- “Synthesis and Biological Evaluation of 2,3’-Diindolylmethanes as Agonists of Aryl Hydrocarbon Receptor.” Winston-McPherson, G. N.; Shu, D.; Tang, W.* Bioorg. Med. Chem. Lett. 2014, 24, 4023-4025. Link.
- “Cinchona Alkaloids as Organocatalysts in Enantioselective Halofunctionalization of Alkenes and Alkynes.” Zheng, S.; Schienebeck, C. M.; Zhang, W.; Wang, H.-Y.; Tang, W.* Asian J. Org. Chem. 2014, 3, 366-376. (Invited review) Link.
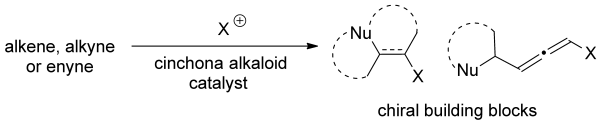
- “Intermolecular bromoesterification of conjugated enynes: an efficient synthesis of bromoallenes.” Wang, H.-Y.; Zhang, W.; Schienebeck, C. M.; Bennett, S. R.; Tang, W.* Org. Chem. Front. 2014, 1, 386-390. (Invited contribution) Link.
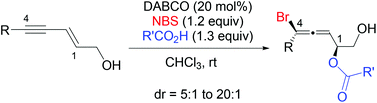
- “3-Acyloxy-1,4-enyne: a New Five-Carbon Synthon for Rhodium-Catalyzed [5 + 2] Cycloadditions.” Schienebeck, C. M.; Li, X.; Shu, X.-Z.; Tang, W.* Pure Appl. Chem. 2014, 86, 409-417. (Invited review) Link.
- “Tethered Spectroscopic Probes Estimate Dynamic Distances with Subnanometer Resolution in Voltage-Dependent Potassium Channels.” Jarecki, B. W.; Zheng, S.; Zhang, L.; Li, X.; Zhou, X.; Cui, Q.; Tang, W.; Chanda, B.* Biophysical J. 2013, 105, 2724-2732. Link. Highlight in Nature Chemical Biology Link.
- “Rhodium-Catalyzed Tandem Annulation and (5+1) Cycloaddition: 3-Hydroxy-1,4-enyne as the 5-Carbon Component.” Li, X.; Song, W.; Tang, W.* J. Am. Chem. Soc. 2013, 135, 16797-16800. Link.
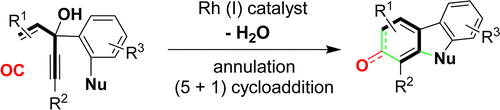
- “Transfer of Chirality in the Rhodium-Catalyzed Intramolecular [5+2] Cycloaddition of 3-Acyloxy-1,4-Enynes (ACEs) and Alkynes: Synthesis of Enantioenriched Bicyclo[5.3.0]decatrienes.” Shu, X.-Z.; Schienebeck, C. M.; Song, W.; Guzei, I. A.; Tang, W.* Angew. Chem. Int. Ed. 2013, 52, 13601-13605. Link.
Highlight in Synfacts, 2014, volume 10, issue 3, 0295 (doi:10.1055/s-0033-1340687)
![Graphical abstract for Tang article "Transfer of Chirality in the Rhodium-Catalyzed Intramolecular [5+2] Cycloaddition of 3-Acyloxy-1,4-Enynes (ACEs) and Alkynes: Synthesis of Enantioenriched Bicyclo[5.3.0]decatrienes"](https://pharmacy.wisc.edu/faculty/tang-research-group/wp-content/uploads/sites/10/2024/09/tang-pub34.gif)
- “Stereoselective Halocyclization of Alkenes with N-Acyl Hemiaminal Nucleophiles.”
Liu, N.; Wang, H.-Y.; Zhang, W.; Jia, Z.-H.; Guzei, I. A.; Xu, H.-D.;* Tang, W.* Chirality 2013, 25, 805-809. Link. (Invited contribution.) - “Stereoselective Total Synthesis of Hainanolidol and Harringtonolide via Oxidopyrylium-Based [5+2] Cycloaddition.” Zhang, M.; Liu, N.; Tang, W.* J. Am. Chem. Soc. 2013, 135, 12434-12438. Link.
![Graphical abstract image for Tang pub "Stereoselective Total Synthesis of Hainanolidol and Harringtonolide via Oxidopyrylium-Based [5+2] Cycloaddition"](https://pharmacy.wisc.edu/faculty/tang-research-group/wp-content/uploads/sites/10/2024/09/tang-pub32.gif)
- “Platinum-Catalyzed Tandem Indole Annulation/Arylation for the Synthesis of Diindolylmethanes and Indolo[3,2-b]carbazoles.” Shu, D.; Winston-McPherson, G. N.; Song, W.; Tang, W.* Org. Lett. 2013, 15, 4162-4165. Link.
![Graphical abstract image for Tang pub "Platinum-Catalyzed Tandem Indole Annulation/Arylation for the Synthesis of Diindolylmethanes and Indolo[3,2-b]carbazoles"](https://pharmacy.wisc.edu/faculty/tang-research-group/wp-content/uploads/sites/10/2024/09/tang-pub31.gif)
- “Rh-Catalyzed (5+2) Cycloadditions of 3-Acyloxy-1,4-enynes and Alkynes: Computational Study of Mechanism, Reactivity, and Regioselectivity.” Xu, X.;* Liu, P.; Shu, X.-Z.; Tang, W.*; Houk, K. N.* J. Am. Chem. Soc. 2013, 135, 9271-9274. Link.
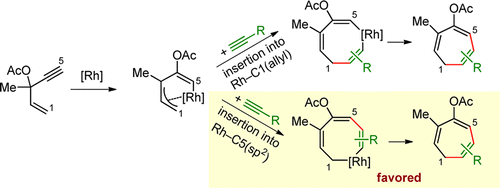
- “Generation of Rhodium(I) Carbenes from Ynamides and Their Reactions with Alkynes and Alkenes.” Liu, R.; Winston-McPherson, G. N.; Yang, Z.-Y.; Zhou, X.; Song, W.; Guzei, I. A.; Xu, X.;* Tang, W.* J. Am. Chem. Soc. 2013, 135, 8201–8204. Link.
- “Stereoselective Addition of Halogen to Conjugated Enynes and Its Application in the Total Synthesis of (-)-Kumausallene.” Werness, J. B.; Zhang, W.; Tang, W.* in Strategies and Tactics in Organic Synthesis Ed., Harmata, M., Elsevier Science, Pergamon Press: Oxford, UK 2013, Vol. 9, Chapter 10, pp 275-291.
- “Enantioselective intermolecular bromoesterification of allylic sulfonamides.”
Zhang, W.; Liu, N.; Schienebeck, C. M.; Zhou, X.; Izhar, I. I.; Guzei, I. A.; Tang, W.* Chem. Sci. 2013, 4, 2652-2656. Link.
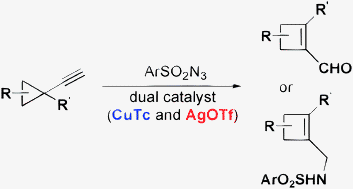
- “Ring Expansion of Alkynyl Cyclopropanes to Highly Substituted Cyclobutenes via a N-Sulfonyl-1,2,3-Triazole Intermediate.” Liu, R.; Zhang, M.; Winston-McPherson, G.; Tang, W.* Chem. Commun. 2013, 49, 4376-4378. Link.
(This article is part of the ChemComm ‘Emerging Investigators 2013’ themed issue.)
- “Effect of Ester on Rhodium-Catalyzed Intermolecular [5 + 2] Cycloaddition of 3-Acyloxy-1,4-enynes and Alkynes.” Schienebeck, C. M.; Robichaux, P. J.; Li, X.; Chen, L.; Tang, W.* Chem. Commun. 2013, 49, 2616-2618. Link.
![Graphical abstract image for Tang article "Rhodium- and Platinum-catalyzed [4+3] Cycloaddition with Concomitant Indole Annulation: Synthesis of Cyclohepta[b]indoles"](https://pharmacy.wisc.edu/faculty/tang-research-group/wp-content/uploads/sites/10/2024/09/tang-pub25.gif)
- “Rhodium- and Platinum-catalyzed [4+3] Cycloaddition with Concomitant Indole Annulation: Synthesis of Cyclohepta[b]indoles.” Shu, D.; Song, W.; Li, X.; Tang, W.* Angew. Chem. Int. Ed. 2013, 52, 3237-3240. Link.
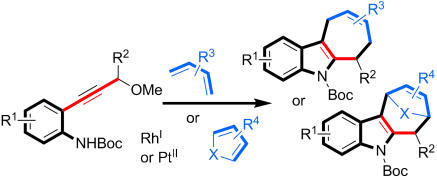
- “Rhodium-Catalyzed Chemo- and Regioselective Cross-Dimerization of Two Terminal Alkynes.” Xu, H.-D.*; Zhang, R.-W.; Li, X., Huang, S., Tang, W.; Hu, W.-H. Org. Lett. 2013, 15, 840-843. Link.
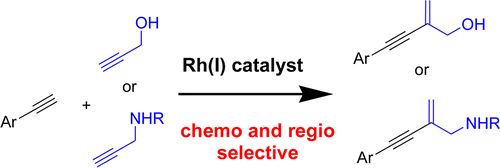
Publications 2007-2012
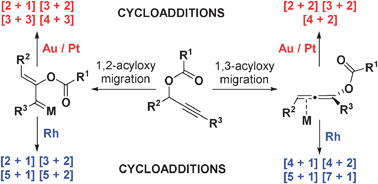
- “Rhodium-catalyzed acyloxy migration of propargylic esters in cycloadditions, inspiration from the recent “gold rush.” Shu, X.-Z.; Shu, D.; Schienebeck, C. M.; Tang, W.* Chem. Soc. Rev. 2012, 41, 7698-7711. Link.
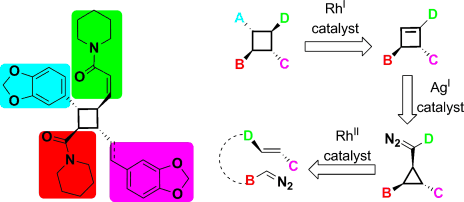
- “Rhodium-Catalyzed Carbonylation of Cyclopropyl Substituted Propargyl Esters: A Tandem 1,3-Acyloxy Migration [5 + 1] Cycloaddition.” Shu, D.; Li, X.; Zhang, M.; Robichaux, P. J.; Guzei, I. A.; Tang, W.* J. Org. Chem. 2012, 77, 6463-6472. Link.
![Graphical abstract for Tang pub "Rhodium-Catalyzed Carbonylation of Cyclopropyl Substituted Propargyl Esters: A Tandem 1,3-Acyloxy Migration [5 + 1] Cycloaddition"](https://pharmacy.wisc.edu/faculty/tang-research-group/wp-content/uploads/sites/10/2024/09/tang-pub21.gif)
- .”Synthesis of Functionalized Cyclohexenone Core of Welwitindolinones via Rhodium-Catalyzed [5 + 1] Cycloaddition.” Zhang, M.; Tang, W.* Org. Lett. 2012, 14, 3756-3759. Link.
![Graphical abstract image for Tang pub "Synthesis of Functionalized Cyclohexenone Core of Welwitindolinones via Rhodium-Catalyzed [5 + 1] Cycloaddition"](https://pharmacy.wisc.edu/faculty/tang-research-group/wp-content/uploads/sites/10/2024/09/tang-pub20.gif)
- “Stereoselective Preparation of Cyclobutanes with Four Different Substituents: Total Synthesis and Structural Revision of Pipercyclobutanamide A and Piperchabamide G.”
Liu, R.; Zhang, M.; Wyche, T. P.; Winston-McPherson, G. N.; Bugni, T. S.; Tang, W.* Angew. Chem. Int. Ed. 2012, 51, 7503-7506. Link. Highlight in Nature Chemical Biology Link.
- “Catalytic Enantioselective Halolactonization of Enynes and Alkenes.” Zhang, W.; Liu, N.; Schienebeck, C. M.; Decloux, K.; Zheng, S. Werness, J. B.; Tang, W.* Chem.-Euro. J. 2012, 18, 7296-7305. Link.
Highlight in Synfacts, 2012, issue 7, 0790 (doi:10.1055/s-0031-1289837).
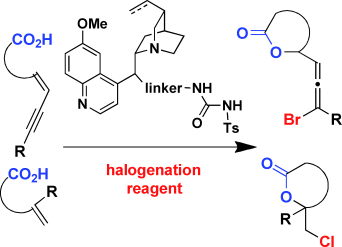
- “Rhodium-catalyzed Intra- and Intermolecular [5+2] Cycloaddi-tion of 3-Acyloxy-1,4-enyne and Alkyne with Concomitant 1,2-Acyloxy Migration.” Shu, X.-z; Li, X.; Shu, D.; Huang, S.; Schienebeck, C. M.; Zhou, X.; Robichaux, P. J.; Tang, W.* J. Am. Chem. Soc. 2012, 134, 5211-5221. Link.
![Graphical abstract image for Tang pub "Rhodium-catalyzed Intra- and Intermolecular [5+2] Cycloaddi-tion of 3-Acyloxy-1,4-enyne and Alkyne with Concomitant 1,2-Acyloxy Migration"](https://pharmacy.wisc.edu/faculty/tang-research-group/wp-content/uploads/sites/10/2024/09/tang-pub17.gif)
- “Rhodium-Catalyzed Carbonylation of 3-Acyloxy-1,4-enynes for the Synthesis of Cyclopentenones.” Li, X.; Huang, S.; Schienebeck, C. M.; Shu, D.; Tang, W.* Org. Lett. 2012, 14, 1584-1587. Link.
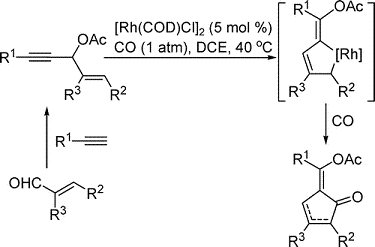
- “Rhodium-catalyzed 1,3-Acyloxy Migration and Subsequent Intramolecular [4+2] Cycloaddition of Vinylallene and Unactivated Alkyne.” Huang, S.; Li, X.; Lin, C. L.; Guzei, I. A.; Tang, W.* Chem. Commun. 2012, 48, 2204-2206. Link.
![Graphical abstract image from Tang pub "Rhodium-catalyzed 1,3-Acyloxy Migration and Subsequent Intramolecular [4+2] Cycloaddition of Vinylallene and Unactivated Alkyne"](https://pharmacy.wisc.edu/faculty/tang-research-group/wp-content/uploads/sites/10/2024/09/tang-pub15.gif)
- “Discovery of histone deacetylase 8 selective inhibitors.” Tang, W.*; Luo, T.; Greenberg, E. F.; Bradner, J. E.; Schreiber, S. L.* Bioorg. Med. Chem. Lett. 2011, 21, 2601-2605. Link.
- “Effect of Halogenation Reagents on Halocyclization and Overman Rearrangement of Allylic Trichloroacetimidates.” Liu, N.; Schienebeck, C. M.; Collier, M. D.; Tang, W.* Tetrahedron Lett. 2011, 52 , 6217-6219. Link.
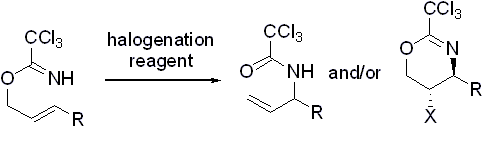
- “Rhodium-catalyzed Ring Expansion of Cyclopropanes to Seven-membered Rings by 1,5-C-C Bond Migration.” Li, X.; Zhang, M.; Shu, D.; Robichaux, P. J.; Huang, S.; Tang, W.* Angew. Chem. Int. Ed. 2011, 50, 10421-10424. Link
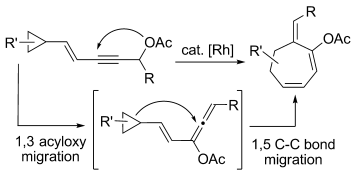
- “Interception of a Rautenstrauch Intermediate by Alkynes for [5+2] Cycloaddition: Rhodium-Catalyzed Cycloisomerization of 3-Acyloxy-4-ene-1,9-diynes to Bicyclo[5.3.0]decatrienes.” Shu, X.-z.; Huang, S.; Shu, D.; Guzei, I. A.; Tang, W.* Angew. Chem. Int. Ed. 2011, 50, 8153-8156. Link.
Highlighted as “hot paper”
![Graphical abstract for Tang pub "Interception of a Rautenstrauch Intermediate by Alkynes for [5+2] Cycloaddition: Rhodium-Catalyzed Cycloisomerization of 3-Acyloxy-4-ene-1,9-diynes to Bicyclo[5.3.0]decatriene"](https://pharmacy.wisc.edu/faculty/tang-research-group/wp-content/uploads/sites/10/2024/09/tang-pub12.gif)
- “Stereoselective Total Synthesis of (-)-Kumausallene.” Werness, J. B.; Tang, W.* Org. Lett. 2011, 13, 3664-3666. Link.
Highlight in Synfacts, 2011, issue 10, 1042 (doi:10.1055/s-0030-1261141).
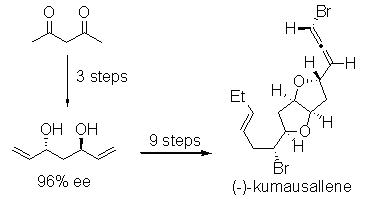
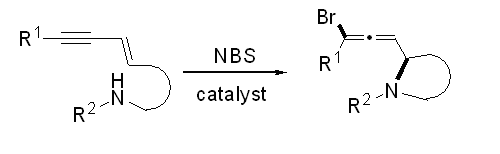
- “Intramolecular 1,4-Addition of Nitrogen Nucleophile and Bromine Electrophile to Conjugated 1,3-Enyne.” Liu, N.; Werness, J. B.; Guzei, I. A. Tang, W.* Tetrahedron 2011, 67, 4385-4390. Link
(Invited contribution for F. Dean Toste’s’ Tetrahedron Young Investigator Award.)
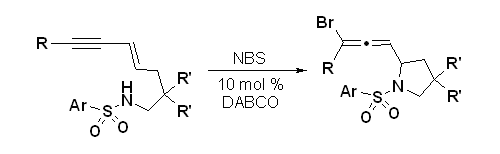
- “Synthesis of Highly Functionalized Cyclohexenone Rings: Rhodium-Catalyzed 1,3-Acyloxy Migration and Subsequent [5+1] Cycloaddition.” Shu, D.; Li, X.; Zhang, M.; Robichaux, P. J.; Tang, W.* Angew. Chem. Int. Ed. 2011, 50, 1346-1349. Link.|
![Graphical abstract image for Tang pub "Synthesis of Highly Functionalized Cyclohexenone Rings: Rhodium-Catalyzed 1,3-Acyloxy Migration and Subsequent [5+1] Cycloaddition"](https://pharmacy.wisc.edu/faculty/tang-research-group/wp-content/uploads/sites/10/2024/09/tang-pub9.gif)
- “Synthesis of bromoallenyl pyrrolidines via 1,4-addition to 1,3-enynes.” Werness, J. B.; Tang, W.* Sci. China Chem. 2011, 54, 56-60. Link.
(Invited contribution for the 6th Sino-US Chemistry Professor Conference at Hangzhou, China.)
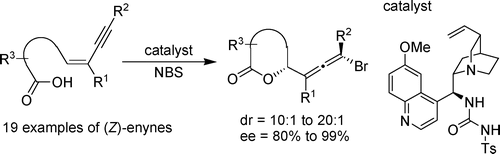
- “Enantioselective Bromolactonization of Conjugated (Z)-Enynes.” Zhang, W.; Zheng, S.; Liu, N.; Werness, J. B.; Guzei, I. A.; Tang, W.* J. Am. Chem. Soc. 2010, 132, 3664-3665. Link.
Highlight in Angew. Chem. Int. Ed. Link.
- “Thermodynamic Control of the Electrocyclic Ring Opening of Cyclobutenes: C=X Substituents at C-3 Mask the Kinetic Torquoselectivity.” Um, J. M.; Xu, H.-D.; Houk, K. N.*; Tang, W.* J. Am. Chem. Soc. 2009, 131, 6664-6665. Link.
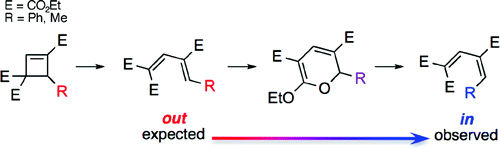
- “DABCO-Catalyzed 1,4-Bromolactonization of Conjugated Enynes: Highly Stereoselective Formation of a StereogenicCenter and an Axially Chiral Allene.” Zhang, W.; Xu, H.-D.; Xu, H.; Tang, W.* J. Am. Chem. Soc. 2009, 131, 3832-3833. Link.
Highlight in Synfacts, 2009, issue 6, 0604 (doi:10.1055/s-0029-1216700).
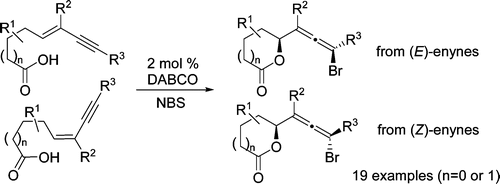
- “Intramolecular Hydroamination of Conjugated Enynes.” Zhang, W.; Werness, J. B.; Tang, W.* Tetrahedron 2009, 65, 3090-3095. Link.
(Invited contribution for Justin DuBois’ Tetrahedron Young Investigator Award.)
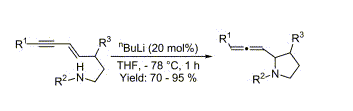
- “N,N’-(11S,12S)-(9,10-dihydro-9,10-ethanoanthracene-11,12-diyl)bis[2-(diphenylphosphino)-Benzamide.” Tang, W. The Encyclopedia of Reagents for Organic Synthesis [EROS], (Ed. P. L. Fuchs, John Wiley and Sons) 2008.
- “Synthesis of Cyclobutenes by Highly Selective Transition-Metal-Catalyzed Ring Expansion of Cyclopropanes.” Xu, H.-D.; Zhang, W.; Shu, D.; Werness, J. B.; Tang, W.* Angew. Chem. Int. Ed. 2008, 47, 8933-8936. Link .
Highlight in Synfacts, 2009, issue 1, 0063 (doi:10.1055/s-0028-1087400).
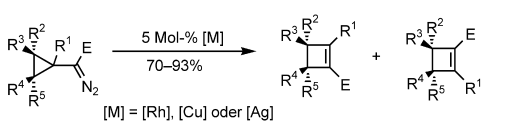
- “Base-Catalyzed Intramolecular Hydroamination of Conjugated Enynes.” Zhang, W.; Werness, J. B.; Tang, W.* Org. Lett. 2008, 10, 2023-2026. Link
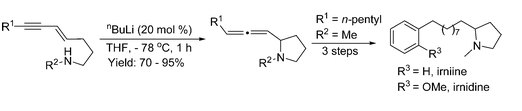
- “Rhodium-catalyzed acyloxy migration of propargylic esters in cycloadditions, inspiration from the recent “gold rush.” Shu, X.-Z.; Shu, D.; Schienebeck, C. M.; Tang, W.* Chem. Soc. Rev. 2012, 41, 7698-7711. Link.