Antibiotic resistance or tolerance to primary anti-Gram-positive therapies, such as β-lactams, vancomycin, and daptomycin often leads to poor clinical outcomes and dramatic increases in health-care expenditures. Approximately 30% of patients with Staphylococcus aureus bacteremia and exhibit primary therapeutic failures, with in-hospital mortality rates approaching 20% despite receiving seemingly appropriate antimicrobial therapy. In addition, persistent bacteremias, despite in vitro susceptibility profiles, remains a clinical challenge, with few available alternative therapies. This has lead to the investigation of “older antimicrobials”, which although traditionally active, may have promising clinical utility in combination with newer antibiotics.
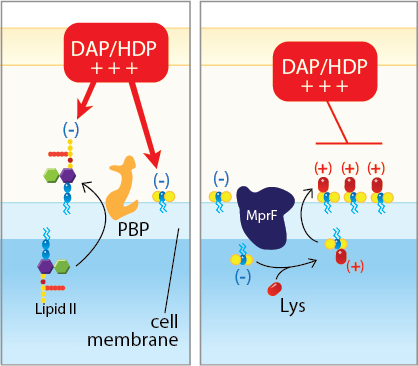
The focus of the Rose Laboratory is combining the older antibiotics with primary agents vancomycin or daptomycin. Interesting, when resistance develops to these primary agents both in vitro and in the clinical, a collateral effect of enhanced sensitivity to older agents occurs. We and others have surprising found that β-lactam antibiotics, traditionally inactive against methicillin-resistant Staphylococcus aureus, synergize with primary agents. Our research focuses on the pharmacodynamic synergy as well as mechanisms involved in this enhanced antimicrobial effect. This is noted two ways (1) combinatorial synergy and (2) prevention of the emergence resistance to vancomycin and daptomycin (see Fig. 1). We have identified that penicillin-binding proteins (PBPs) play an essential role in the bacterial compensatory response to daptomycin, and that selective inhibition of PBPs leads to significantly enhanced killing by a dual mechanism effect. β-lactam antibiotics also uniquely prevent the emergence of daptomycin resistance, primarily by inhibiting the first step genetic mutation found in many daptomycin-resistant strains. Collectively, these findings have begun to have direct patient application.
Funding: NIH/NIAID R01AI132627

