Antibiotics continue to play an essential role in treating bacterial infections, and underlying mechanisms of bacterial killing by different antibiotics are established. However, it is not understood how and when these antibiotics regulate gene expression and production of toxins and adhesins, particularly when used in a combination therapy approach and during the infection process. A number of mechanisms have been proposed to explain antimicrobial resistance in bacterial biofilms. Organisms within a biofilm have significantly slower growth than planktonic cells, reducing the activity of antibiotics targeting cell wall synthesis and division. Meanwhile, bacterial exotoxins can be stimulated or suppressed upon antibiotic treatment, which can affect patient response.
We use a number of different in vitro systems to investigate the effect of antimicrobials on bacterial virulence and biofilm production. These exposures range from “static” systems where antimicrobial concentration does not change, to “dynamic” systems designed to mimic human pharmacokinetic exposures. We have investigated the latter in the hollow fiber pharmacodynamic model system (see figure), which allows for simulated antimicrobial exposure and sequestering of bacteria and bacterial products for assessment. Our studies have found that the effect of antimicrobials on Staphylococcus aureus toxins are strain specific, and have confirmed that antibiotics that target protein synthesis inhibit toxin production. Importantly, we have identified a time from the initiation of therapy where this inhibition is significant, around 8 hours post-dose. Some commonly used drugs, such as trimethoprim/sulfamethoxazole actual stimulate excessive toxin release, which is known to be deleterious to patients.
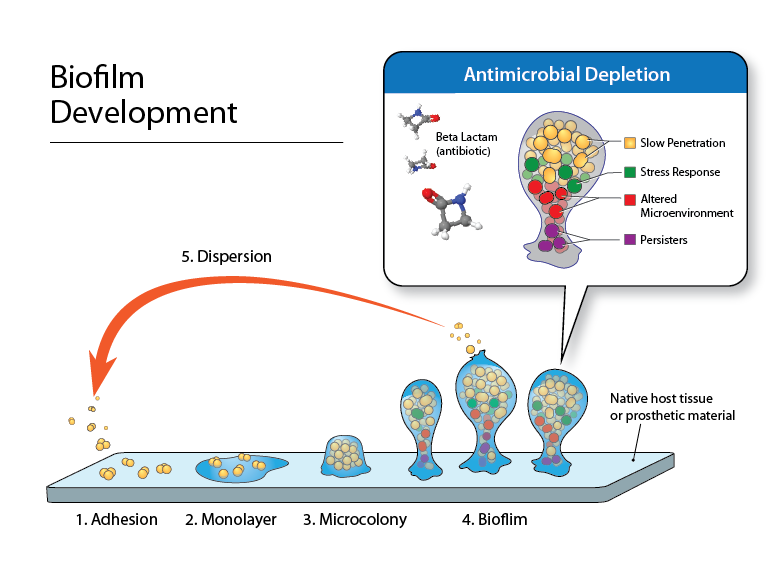
Staphylococcus aureus biofilms are inherently heterogeneous in production and composition. Our studies are using models to grow bacterial biofilms and exposure them to antimicrobial combinations. In addition, we collaborate with multiple groups to test new antimicrobial formulations and novel compounds for anti-biofilm activity in S. aureus as well as other organisms such as Pseudomonas aeruginosa, which produce complex and difficult-to-treat biofilms.
Funding:
NIH/NIAID R21 AI121704 (PI: J. Kwan)
Merck
Theravance Biopharma

