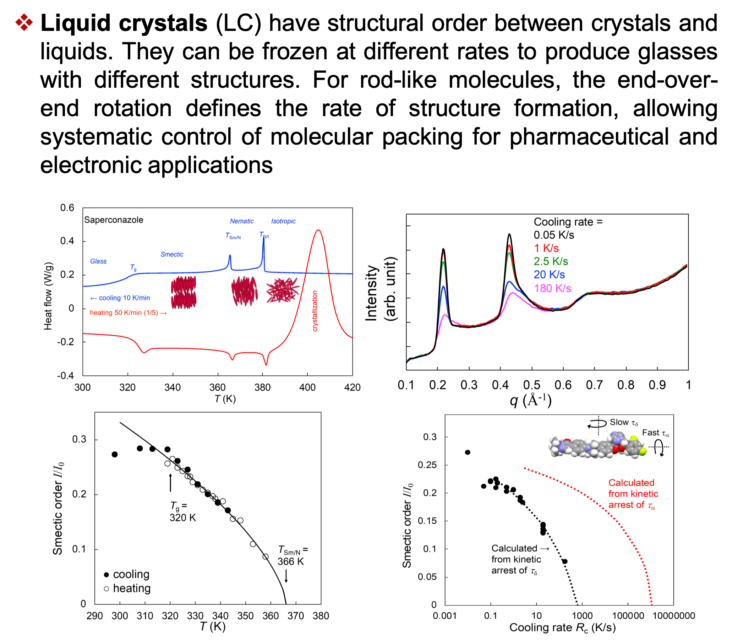
Overview
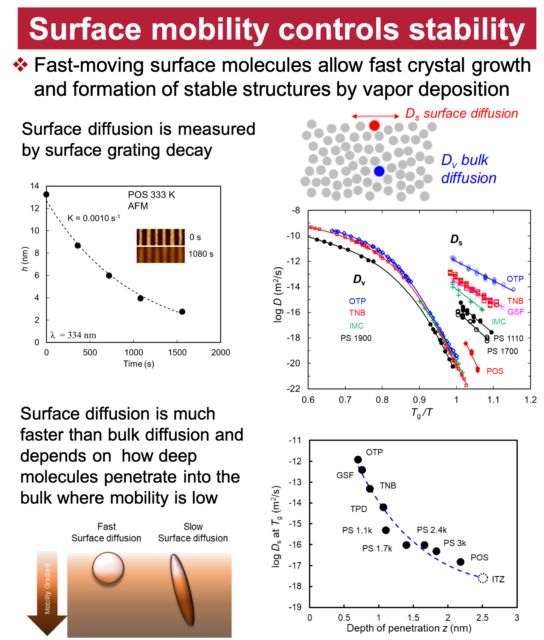
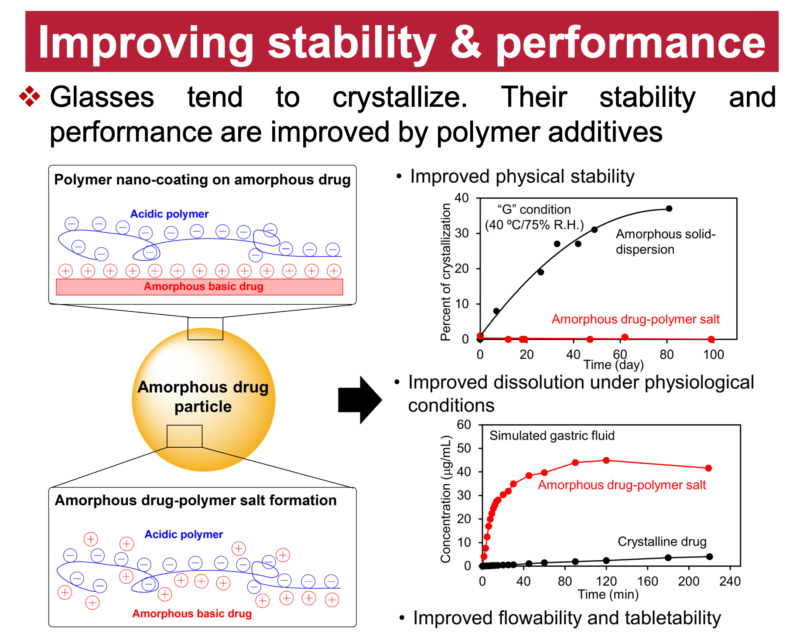
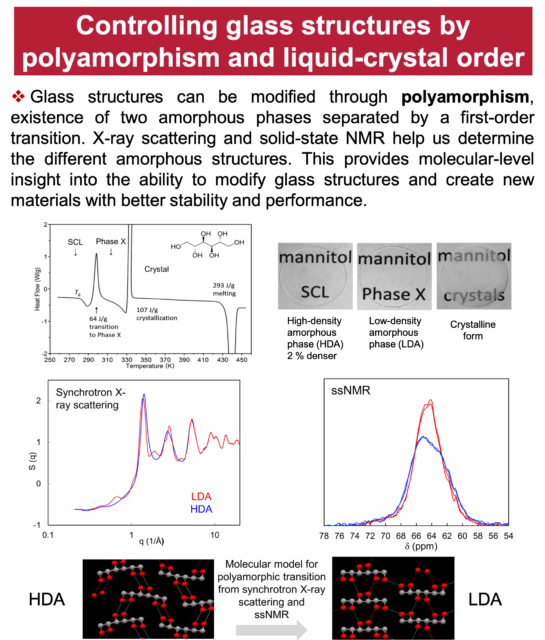
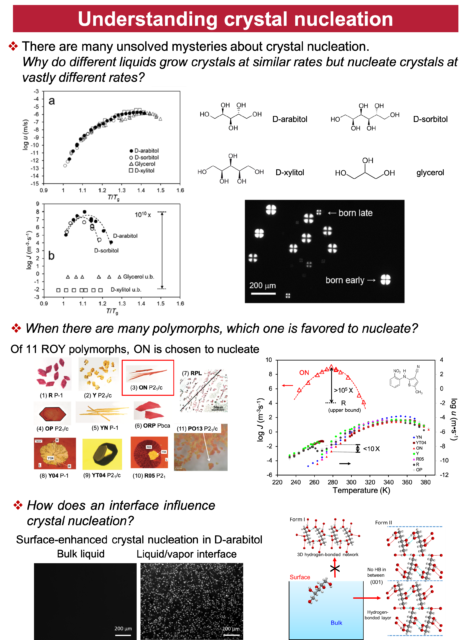
We study the solids of organic molecules, with special attention to the different solids of the same molecules. A key property of solids is the ability of the same substance to form different and long-lasting structures. Carbon can crystallize as diamond and graphite; silicon dioxide can solidify as quartz (crystalline) and glass (amorphous). The discovery of new solid forms provides new materials (e.g., C60 and carbon nanotubes) and new knowledge of materials. Our study of organic solids is also motivated by their importance in developing drugs, organic electronics, and other soft materials. Recent studies have revealed new and surprising features of organic solids unknown for inorganic solids. In this laboratory, physical measurements and crystallization experiments are combined to understand how different solid forms can result from the same liquid and transform among themselves. Our major techniques are crystallography, calorimetry, spectroscopy, and microscopy.
Funding and Collaboration